
Federal Vaccine Advisory Committee Update
As 2017 unfolds, federal advisory committees - the Advisory Committee on Immunization Practices (ACIP), the National Vaccine Advisory Committee (NVAC), and the Advisory Commission on Childhood Vaccines (ACCV) - will be holding meetings throughout 2017. Over the past few years, the FDA Vaccines and Related Biological Products Advisory Committee (VRBPAC) has been reducing the numbers of times it holds public meetings to discuss new vaccine licensing issues and currently has only one meeting scheduled in 2017.

These federal vaccine advisory committees shape national vaccine policies and influence state vaccine laws and policies. During 2016, each vaccine advisory committees’ meetings were punctuated with discussions on topics such as an HPV vaccine series dose reduction; discontinued use of live attenuated influenza vaccine (LAIV); strategies to insure the success of the National Adult Immunization Plan (NAIP); vaccine development innovation; and the ever increasing vaccine injury caseload for attorneys in the U.S. Department of Justice and Special Masters in the U.S. Court of Claims adjudicating the federal vaccine injury compensation program (VICP).
Though these committees are advisory in nature, the reality is that their recommendations and reports often get turned into state vaccine mandates and policies that negatively impact the availability of vaccine exemptions, as well as threaten consumer privacy and how vaccine exemption data is collected and shared across state and federal databases and with third parties (interoperability). In recent years, many of the recommendations made by these committees have resulted in an increase in the introduction of state bills and policies to restrict voluntary vaccine decision-making.
No State is Safe from Attacks on Vaccine Informed Consent Rights
It is the end of February and in most states, that means that the legislative sessions are underway and new bills are being introduced. The question is not if legislation to restrict your vaccine exemption and information disclosure rights will come to your state; the question is only when. NVIC is already monitoring over 100 vaccine-related bills in more than 30 states.1
The National Vaccine Information Center, now in our 35th year of working to prevent vaccine injuries and deaths through public education, is an information clearinghouse on vaccine science, policy, law and ethics. Among our commitments to the public are providing public comment to and independent oversight on the actions being taken by federal vaccine advisory committees.
This information can be used when action is needed to defend informed consent rights, including protection of vaccine exemptions, but there is no substitute for citizen participation in the law and policy making process.
If you haven’t already registered to be a user of NVIC’s Advocacy Portal so you can stay up-to-date on vaccine bills moving in your state and receive email alerts on critical actions that need to be taken, please sign up now. It is a free public resource that provides analysis, talking points and puts you in contact with your own legislators. It is important that you visit the Advocacy Portal often so you don’t miss any vaccine-related bill moving in your state legislature and can make your voice heard even before NVIC issues an action alert.
DHHS Supports Tracking Vaccine Status of All Adults

While immunization information systems (IIS), better known as vaccine tracking registries, are largely already in place in every state so public health officials can track the vaccination status of all children, chief among the goals of the NVAC’s launch of the National Adult Immunization Plan (NAIP) 2 is to use these registries to also track the vaccination status of all adults. As NVIC previously reported, the NAIP is being launched to increase vaccine uptake in adults. Key strategies in the plan include incentivizing health care professionals to administer up to 11 vaccines to all adults starting at age 19 through 65, in accordance with the Centers for Disease Control’s (CDC) recommended adult vaccine schedule;3 outreach and networking with employers to require vaccination as a condition of employment; and tracking adult vaccine status with state owned registries (IIS) and health information exchange (HIE) databases.
In 2016, the U.S. Department of Health and Human Services (DHHS) Acting Assistant Secretary for Health (ASH) Karen DeSalvo M.D. reaffirmed support for the NAIP. Dr. DeSalvo also reported the tripling of health care providers creating Electronic Health Records (EHR), which are electronic versions of personal health/medical records created by health care professionals. Use of EHRs under “meaningful use” provides the ability for government health officials to target populations for vaccination when needed. During the meeting the acting ASH stated that DHHS would use their “bully pulpit” to increase adult vaccination for emerging diseases like Zika.4
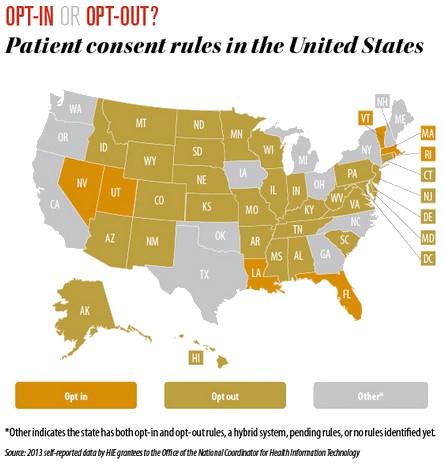
State Vaccine Registries Use Electronic Medical Records
Much like state vaccine registries, rules for state HIE databases that contain personal medical information on Americans differ from state to state.5 Your ability to opt-in or opt-out and/or place restrictions on how your medical, inclusive of vaccine status, is shared in EHRs with third parties and the government is largely decided at the state level.
One of the goals in the NAIP is to automate the transfer of vaccine data from EHRs into state vaccine registries operated by state health departments.6 Removing interoperability barriers that may prevent the transfer of personal medical information, or prevent vaccine data sharing in general is seen by the NVAC as a leverage mechanism to compel all Americans to comply with federal vaccine recommendations.7 This data automation means that any information about your vaccine status that your doctor records in your EHR may end up in your state health department’s vaccine registry and can be shared with third parties without your knowledge or informed consent.
Patient Privacy Is Eroding
Health care providers were paid to create EHRs under the Health Information Technology for Economic and Clinical Health Act of 2009 (HITECH).8 After 2015, health care professionals can be penalized for not implementing EHRs for all patients.9
However, the real danger of the continued erosion of patient privacy posed by EHRs is that EHRs are attractive targets for ransomware,10 11 security breaches,12 and changes to the 1996 Health Insurance Portability and Accountability Act (HIPAA) that allow disclosure of your personal medical records to the government and other third parties.13 The use of EHRs to leverage vaccine compliance is only the latest example of erosions of medical privacy.
NVIC recommends contacting your health care provider with any concerns you have about how your EHR data or your child’s EHR data is shared so that you can take steps to protect your medical privacy. You may also use the vaccine registry links NVIC provides on its website in the Quick Facts box for each state to learn more about how your state’s vaccine registry operates and whether you have the right to opt-in or opt-out of participating in the registry.
Pregnant Women Reclassified for Research Purposes
2016 NVAC meeting discussions also waded into the possible use of existing maternal research data collected in various research efforts to create a research data repository on pregnant women. The repository would allow records collected with informed consent from pregnant women for use in one research study to be used in a different research studies without the women’s additional consent.

Another subject involving ethics that was discussed by the NVAC was whether or not to redefine pregnant women as a “scientifically complex population”14 instead of a “vulnerable population,” which is the current definition and provides pregnant women and their fetus special research subject protections.15
Redefining pregnant women in this manner would permit pharmaceutical companies to recruit pregnant women for premarketing clinical research trials. One NVAC member stated concern that pregnant women must be a part of the stakeholder process and allowed input into any redefinition of their status for scientific research purposes. However, the American College of Obstetricians and Gynecologists (ACOG) has issued an opinion supporting use of the new definition by arguing that pregnant women have the ability to render informed consent and protect themselves and, therefore, are not a “vulnerable” population.16
NVIC stressed to the committee during public comment that use of previously collected data for repository purposes without the express permission of the research subject was unethical, a violation of privacy and does require specific advance informed consent. NVIC also affirmed the right of pregnant women to ultimately decide their participation in any scientific research.17
More Vaccines on the Horizon for Pregnant Women
During 2016, the ACIP was advised of efficacy problems with Phase III clinical trials for the Novavax respiratory syncytial virus (RSV) vaccine. The Novavax Phase III trial showed little to no protection in seniors and the company is refocusing development of the vaccine for use in pregnant women.18 There are numerous RSV vaccines under development19 and it seems likely that an RSV vaccine candidate will be licensed in at some point in the near future.
RSV infects most children before their second birthday, with the primary symptoms of RSV resembling those of the common cold, and most RSV infections resolve without complications. However, for a small percentage of children, RSV can cause bronchiolitis and be life-threatening. Those at greater risk of severe infection are premature infants, children born with heart or lung disease, babies with weakened immune systems and children under eight weeks of age.20
Safety Data on Maternal Tdap Vaccination Is Limited
Information on the safety of Tdap vaccine use in pregnant women was also presented to the ACIP. The CDC recommends that all pregnant women receive a booster dose of Tdap vaccine in the third trimester of every pregnancy and this is an off-label recommendation, due to the fact that Tdap was never approved by the FDA for use in pregnant women.21 Although the government maintains that no significant health problems have been proven to be associated with use of Tdap by pregnant women, accurately detecting adverse outcomes is compromised by the lack of valid comparative data on pregnancy outcomes in women who do not get Tdap during pregnancy.22
The NVAC also solicited public comment on the draft report and draft recommendations for overcoming barriers to vaccine uptake and identifying opportunities for developing maternal immunizations.23 The report failed to recognize ethical issues and lack of pre-licensure clinical data on vaccine safety and effectiveness in promoting vaccination of pregnant women. Instead the report focused on how to increase Tdap and influenza vaccine uptake among pregnant women.
NVIC submitted written public comment for consideration by the NVAC, which was critical of maternal vaccination promotion in the absence of baseline data. NVIC’s public comment also was critical of the NVAC’s support of metrics that compel health care providers to vaccinate pregnant women according to federal vaccine policy rather than acknowledging a professional obligation to respect an individual’s informed consent rights, values and beliefs, medical history and individual susceptibilities that increase risks for vaccine harm.24
ACIP Withdraws Live Nasal Flu Vaccine Recommendation
Based on the CDC’s finding that the live attenuated (nasal) influenza vaccine (LAIV) has not been effective for the past three years, the ACIP voted against recommending use of LAIV for the 2016-2017 flu season. The CDC estimated LAIV effectiveness to be about three percent and concluded that there was no protection provided by LAIV.25
ACIP affirmed the current recommendations for use of inactivated, injectable influenza vaccine.
HPV Vaccine Schedule Reduced to Two Doses
Several past ACIP meetings have involved discussions of Human Papilloma Virus (HPV) vaccine effectiveness data that suggests giving two doses is as effective as giving the originally recommended three doses.
The ACIP voted during their October 2016 meeting to approve a two-dose HPV vaccine schedule for adolescents, which is reflected in the CDC’s 2017 recommended childhood vaccine schedule.26
Flu Shot Injury Petition Meets Uphill Battle
During the ACCV’s June 2016 meeting, the committee heard a rare petition request from the public to add more influenza vaccine injuries to the Vaccine Injury Table (VIT).27 For many of the requested additions, the vaccine injuries were either already a part of the VIT, or are soon to be added. However, for the remaining petitioned conditions of Multiple Sclerosis (MS), and myelitis/transverse myelitis (TM) federal officials advised the ACCV that there was a lack of science supporting influenza vaccine as a cause for these conditions and the ACCV voted against adding these conditions to the VIT.28
In a public comment to ACCV, NVIC noted that information provided to the committee for this petition did not include historical data on influenza vaccine injury awards made in the VICP associated with multiple sclerosis and transverse myelitis. Similar VICP historical data information was provided to the ACCV when considering adding Guillain Barre Syndrome (GBS) to the VIT for influenza vaccine, which prompted federal officials and the ACCV to make a policy decision to include GBS as an influenza vaccine injury on the VIT.29
NVIC requested that as a matter of transparency, future discussions of public VIT petitions should include similar historical data on VICP award information and supporting testimony/science that was submitted by plaintiff’s attorneys.
Vaccine Injury Compensation Flat Compared to Increase in Claims Awarded
During the December 2015 ACCV meeting, the US Department of Justice reported that a successful VICP petitioner had initiated an appeal of their original vaccine injury compensation award from the VICP. The purpose of the appeal was to increase the amount of the original compensation awarded to adequately to meet the financial needs of the vaccine injured individual.30
As revealed by the federally commissioned Altarum report provided to ACCV in 2009,31 a significant number VICP petitioners who have received compensation have voiced similar concerns relating to VICP compensation awards being inadequate to cover the costs to care for a vaccine injured person. The Altarum report also noted that no mechanism was in place to measure satisfaction with the VICP and recommended the creation of an ongoing process for evaluating satisfaction.
During the June 2016 ACCV meeting, it was reported that greater numbers of petitions were being processed through the VICP in a shorter amount of time, while the overall payout amount from the trust fund remains about the same as previous years with fewer cases processed. ACCV Commissioner Kraus, a vaccine injury attorney, expressed concern that this trend may indicate a rush to settle petitions, which may result in inadequate compensation to the vaccine injured.
During public comment, NVIC echoed Commissioner Kraus' concerns and renewed its request to the ACCV to revisit the recommendations made by the Altarum report to create a process that would measure VICP petitioner satisfaction. Such an effort could identify why petitioners are dissatisfied and potentially provide incentive to the VICP to take steps to assure that adequate compensation is awarded to vaccine injured victims.32
How to Learn About Federal Advisory Committees
All of these federal vaccine advisory committees accept public comment and most will accept comments live over the phone. Recommendations from these committees often translate into state legislation to mandate vaccines and/or restrict vaccine exemptions. You can learn more about these committees and when they meet, as well as read NVIC’s public comments here. NVIC encourages the public to engage with these committees.
Change Depends on You Taking Action at the Local Level
Information provided in federal vaccine advisory committee meetings can be used when action is needed to defend informed consent rights, including protection of vaccine exemptions. If you haven’t already registered to be a user of NVIC’s Advocacy Portal, register today so you can stay up-to-date on important vaccine bills moving in your state (or on Capitol Hill) and receive email alerts on critical actions that need to be taken.
Your participation will help NVIC help you preserve your ability to voluntarily accept, delay, or decline vaccines for yourself or your minor child without being sanctioned and you will learn what you can do to protect your personal vaccine information from being shared with third parties, including local, state and federal agencies.
If you don’t know who your elected representatives are at the state and federal level and have not contacted them, we encourage you to take time to get to know your legislators and build a relationship with them. Having these relationships and educating your state legislators on the importance of securing and protecting flexible vaccine exemptions is critical to defending vaccine freedom of choice in America.
Please do not wait for legislation to be introduced in your state before developing this relationship. Consider going to your legislator’s morning coffee sessions and town hall meetings, or make an appointment to have a personal conversation. Share your vaccine safety and informed consent concerns and why it is important to you that they uphold the human right to informed consent to voluntarily accept, delay, or decline one or more vaccinations for yourself or your child.
Not sure where to start? NVIC’s Reforming Vaccine Policy & Law: A Guide was designed for this reason. It is a well referenced vaccine information resource and a good starting point for your conversation.
It’s your health, your family, and your choice - choose to participate!
References:
1 Richardson D. NVIC Tracking 103 Bills in 30 States.
NVIC Newsletter Feb. 8, 2017.
2 Department of Health & Human Services (DHHS). National Vaccine Program Office (NVPO). “Goal 1: Strengthen the Adult Immunization Infrastructure,” Feb. 5, 2016.
3 CDC. Adult Immunization Schedule.
4 DHHS. NVPO. National Vaccine Advisory Committee (NVAC). Certified Meeting Minutes. Feb. 2-3, 2016.
5 K. Terry. Health information exchanges introduce patient consent questions.Medical Economics. Jul. 8, 2014
6 DHHS. NVPO. “Goal 1: Strengthen the Adult Immunization Infrastructure,” Feb. 5, 2016.
7 DHHS. NVPO. NVAC. Presentation by D. Chrysler, JD - University of Michigan School of Public Health. “Addressing Law to Share IIS Data Among States” Slide 3. Feb. 10, 2015.
8 HealthIT.gov. EHR Incentive Programs. Jan. 15, 2013.
9 HealthIT.gov. Are there penalties for providers who don’t switch to electronic health records (EHR)? Jan. 13, 2013
10 J. Davis. Ransomware: See the 14 hospitals attacked so far in 2016. HealthcareITNews. Oct. 5, 2016.
11 J. Davis. Emory Healthcare hit by ransomware, data of over 200,000 patients hacked. HealthcareITNews. Jan. 6, 2017.
12 DHHS. Office of Civil Rights. Breach Portal.
13 Patient Privacy Rights. Did you know that HIPAA enables the use and disclosure of your medical records?
14 DHHS. NVAC Minutes. Jun. 7-8, 2016.
15 UCI Office of Research. Vulnerable Subject Populations – Pregnant Women, Fetuses and Neonates.
16 American Congress of Obstetricians and Gynecologists. Committee Opinion - Ethical Considerations for Including Women as Research Participants. November 2015.
17 Wrangham T. Oral Public Comment to NVAC. Jun. 7-8, 2016.
18 Bayer A. RSV vaccine fails in clinical trial: Novavax switches focus to vaccinating pregnant women as stock plummets. Inquistir Sep. 27, 2016.
19 National Institutes of Health (NIH). ClinicalTrials.gov. Search Results – RSV Vaccines.
20 Mayo Clinic. Diseases and Conditions – Respiratory Syncytial Virus (RSV). Definition. Jul. 9, 2014.
21 Fisher BL. Vaccination During Pregnancy: Is It Safe?NVIC Newsletter Nov. 9, 2013.
22 Centers for Disease Control (CDC). Immunization Safety Office (ISO) presentation to the Advisory Committee on Immunization Practices (ACIP). P. Moro, MD, MPH. Update on the safety of maternal tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap). Jun. 23, 2015.
23 DHHS. Draft - National Vaccine Advisory Committee (NVAC): Overcoming Barriers and Identifying Opportunities for Developing Maternal Immunizations. Aug. 22, 2016.
24 Fisher BL. Wrangham T. NVIC Written Public Comment. NVAC Phase II Draft Recommendations for Overcoming Barriers and Identifying Opportunities for Developing Maternal Immunizations. Sep. 9 2016.
25 CDC. ACIP votes down use of LAIV for 2016-2017 flu season. Media Statement. Jun. 22, 2016.
26 CDC. CDC recommends only two HPV shots for younger adolescents. Press Release. Oct. 19, 2015
27 HRSA. National Vaccine Injury Compensation Program (VICP) presentation to Advisory Commission on Childhood Vaccines (ACCV). T. Dalle-Tezze, MD. Discussion of Petition to Add Neurologic Injuries to Vaccine injury Table for the Influenza Vaccines. Jun. 3, 2016
28 HRSA. ACCV Minutes. Jun. 3, 2016.
29 HRSA. Presentation to ACCV. A. Calvo, MD, MPH. Updating the Vaccine Injury Table: Guillain - Barré Syndrome (GBS) and Seasonal Influenza Vaccines. Sep. 5, 2013.
30 HRSA. ACCV Minutes. Pg 4 - Kenzora v. HHS. Dec. 5, 2015.
31 Altarum Institute. Determining the Feasibility of Evaluating the National Vaccine Injury Compensation Program. Final Report. Jun. 15, 2009.
32 Ibid.










Leave a comment
Your email address will not be published. Required fields are marked with an *