.png?width=500&height=280&ext=.png)
During 2020, a rule-making process to modify the federal Vaccine Injury Table (VIT) by less than transparent means has unfolded over the course of meetings held by the federal Advisory Commission on Childhood Vaccines (ACCV).
An unscheduled discussion took place, during the ACCV’s March meeting, about confidential correspondence that was sent to each Commissioner by the Department of Health and Human Services (DHHS). The correspondence solicited each Commissioner’s personal comments on a draft Notice of Proposed Rule Making (NPRM) to remove syncope and shoulder injury related to vaccine administration (SIRVA) from the VIT. Dr. Cody Meissner, who was the Chair of the Commission, encouraged Commissioners to respond to the correspondence privately, because he said it would be unlikely that the Commission would reach a consensus.1
Backdoor Attempt to Remove Recognized Vaccine Injuries
 The Chairman’s encouragement that committee members respond privately to DHHS about the draft NPRM is contrary to the National Childhood Vaccine Injury Act of 1986.2 This federal law requires that any changes to the VIT be discussed in open meetings conducted by the ACCV and that their recommendations be made publicly, prior to the DHHS Secretary’s publishing of an NPRM to alter the VIT. The soliciting of private comments by DHHS from Commissioners appears to have been a backdoor attempt to circumvent long standing federal law.3 4 5
The Chairman’s encouragement that committee members respond privately to DHHS about the draft NPRM is contrary to the National Childhood Vaccine Injury Act of 1986.2 This federal law requires that any changes to the VIT be discussed in open meetings conducted by the ACCV and that their recommendations be made publicly, prior to the DHHS Secretary’s publishing of an NPRM to alter the VIT. The soliciting of private comments by DHHS from Commissioners appears to have been a backdoor attempt to circumvent long standing federal law.3 4 5
Commissioners Kain and Howie, who strongly opposed Dr. Meissner’s actions, stated that discussion about changes to the VIT should be undertaken publicly as a committee. They also expressed deep concern about the removal of these injuries without a presentation of evidence that would justify their removal from the VIT.6
NVIC Requests Public Discussions of DHHS Draft NPRM
In addition to concerns expressed by ACCV Commissioners in March, NVIC issued a letter in April7 to DHHS and the ACCV noting the legal requirement of the ACCV to publicly discuss and make recommendations to the DHHS Secretary prior to publishing an NPRM. NVIC also requested that ACCV review the original evidence that was presented when these injuries were added to the VIT by unanimous vote in 2012 and weigh it against any new evidence that would justify the removal of these injuries from the VIT.8
An unscheduled ACCV meeting to discuss DHHS’ proposed removal of SIRVA and syncope from the VIT was held on May 18, 2020, due to pressure from organizations and members of the public injured by vaccines, their attorneys and medical professionals.9
Evidence Base for SIRVA and Syncope
In 2009, DHHS engaged the Institute of Medicine (IOM) to review the epidemiologic, clinical, and biological evidence of adverse health outcomes associated with nine vaccines routinely recommended for children in the CDC’s childhood vaccination schedule.
 The subsequent IOM physician committee report published in 2012 included a review of the evidence relating to reports of vaccine adverse events for syncope and SIRVA. Using their standard assessment, the IOM10 found strong mechanistic evidence11 to support a causal relationship between the injection of vaccines for the injuries of syncope and SIRVA, and did not rule out vaccines themselves as a possible mechanism for these injuries.
The subsequent IOM physician committee report published in 2012 included a review of the evidence relating to reports of vaccine adverse events for syncope and SIRVA. Using their standard assessment, the IOM10 found strong mechanistic evidence11 to support a causal relationship between the injection of vaccines for the injuries of syncope and SIRVA, and did not rule out vaccines themselves as a possible mechanism for these injuries.
DHHS presented the IOM findings and expressed their support of adding these injuries to the VIT at the March 8, 2012 ACCV meeting.12 In turn, the ACCV unanimously voted to recommend that these injuries be added to the VIT.13 As a result, syncope and SIRVA were officially added to the VIT in January of 2017.14
The ACCV’s guiding principles for recommending changes to the VIT states that any change to the table should be evidenced based “and, whenever possible, be made to the benefit of petitioners”.15
SIRVA Now Leading VICP Compensation Awards
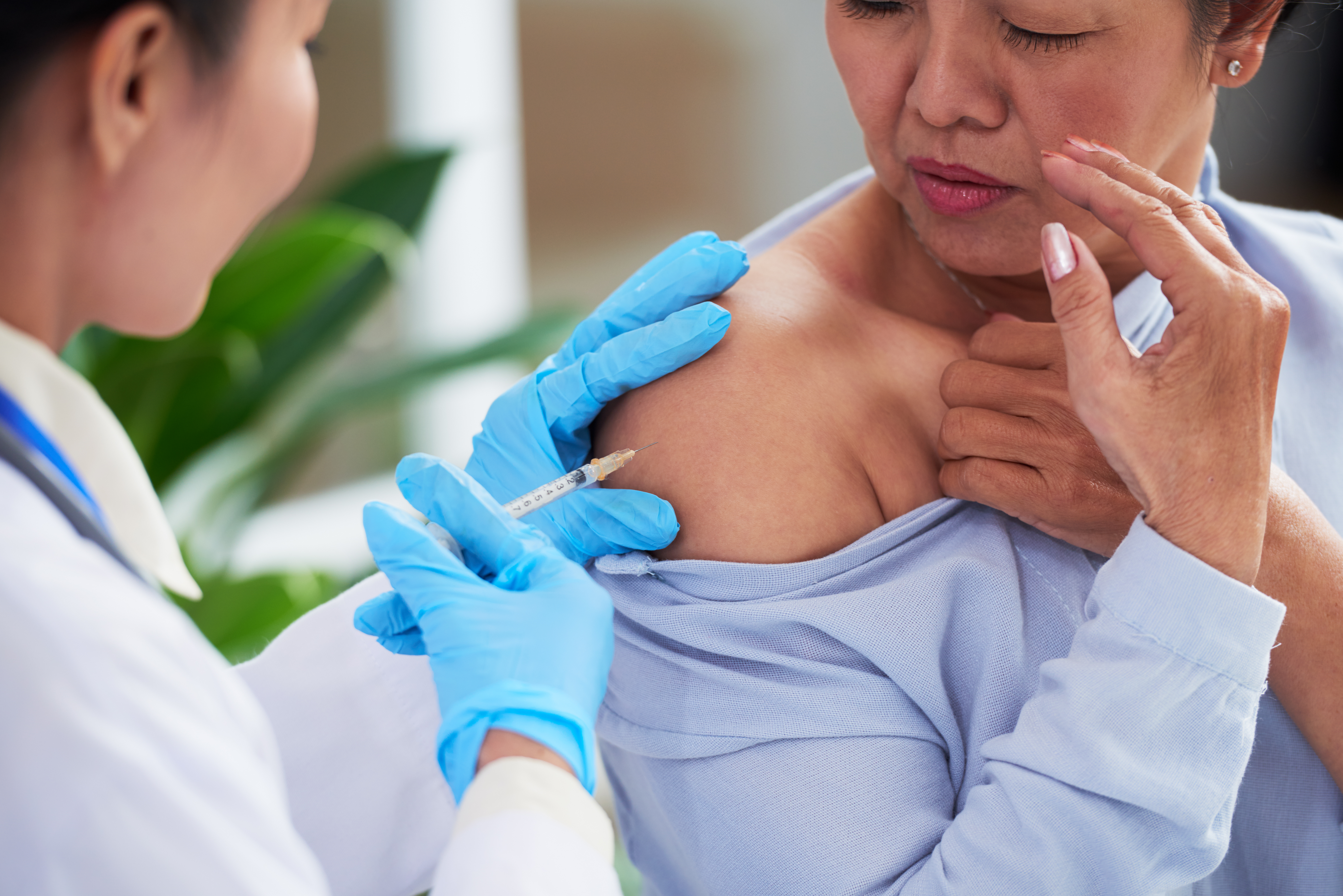 Injury compensation updates presented by DHHS throughout 2020 found that for the past two fiscal years the majority of petitions (54 percent) filed with the VICP were for SIRVA injuries.16 17 18 It was also noted that negotiated settlements have decreased since the addition of SIRVA to the VIT.19 The decrease in the number of SIRVA settlements is evidence that the original intent of the VIT, which was the expeditious compensation of cases resulting from a table of evidenced based injuries, has been met and its removal from the VIT would reverse that accomplishment.
Injury compensation updates presented by DHHS throughout 2020 found that for the past two fiscal years the majority of petitions (54 percent) filed with the VICP were for SIRVA injuries.16 17 18 It was also noted that negotiated settlements have decreased since the addition of SIRVA to the VIT.19 The decrease in the number of SIRVA settlements is evidence that the original intent of the VIT, which was the expeditious compensation of cases resulting from a table of evidenced based injuries, has been met and its removal from the VIT would reverse that accomplishment.
ACCV Unanimously Rejects Removal of Syncope and SIRVA from Federal Vaccine Injury Table
During the ACCV’s May meeting, the ACCV unanimously rejected the proposed removal of syncope and SIRVA from the VIT, largely due to the fact that no new evidence was presented by DHHS to justify their removal.20 During the May, September and December ACCV meetings, Commissioners heard from citizens suffering from SIRVA, the Vaccine Injured Petitioners Bar Association, medical professionals, industry, and non-profit organizations such as NVIC and Vaccinate Your Family (formerly Every Child by Two) who all strongly opposed the removal of syncope and SIRVA from the VIT and requested that recommendations be deferred at least until all current research could presented to the Commission.21
 Mike Milmoe, a vaccine injury attorney, was critical of DHHS’s attempt to remove these injuries from the VIT without presenting supporting evidence, especially in light of the lengthy and detailed process undertaken when these injuries were added to the table. He also found that the U.S. Department of Justice’s (DOJ) letter supporting the removal of these injuries from the VIT, due to the alleged increased workload it creates for DOJ’s staff, had no merit. As a former DOJ attorney in the VICP, Mr. Milmoe noted that by removing these injuries, DOJ staff would actually face increased workloads by having to spend more time trying to negotiate settlements rather than expediting compensation by conceding cases for injuries that appear on the VIT.22
Mike Milmoe, a vaccine injury attorney, was critical of DHHS’s attempt to remove these injuries from the VIT without presenting supporting evidence, especially in light of the lengthy and detailed process undertaken when these injuries were added to the table. He also found that the U.S. Department of Justice’s (DOJ) letter supporting the removal of these injuries from the VIT, due to the alleged increased workload it creates for DOJ’s staff, had no merit. As a former DOJ attorney in the VICP, Mr. Milmoe noted that by removing these injuries, DOJ staff would actually face increased workloads by having to spend more time trying to negotiate settlements rather than expediting compensation by conceding cases for injuries that appear on the VIT.22
Dr. Ramon Rodriguez, JD, from the Vaccine Injured Petitioners Bar Association, stated that DHHS’ concern that compensation of these injuries would cause depletion of the compensation trust fund was unfounded, and noted that the trust fund had continued to grow and was “healthy” despite compensation awards being made for these recognized injuries.23
John Murphy, General Counsel of Biotechnology Innovation Organization which represents the largest international biotechnology trade association in the world, stated that he supports keeping these injuries on the VIT because the rationale used when they were added to the VIT in 2017 remains largely unchanged.24
Dr. Srikumaran, an Associate Professor of Orthopedic Surgery at Johns Hopkins Shoulder and Sports Medicine, gave his professional opinion based on his clinical experience, that vaccine administration alone could not be responsible for all of the cases of SIRVA and that the vaccine itself must play a role. Both Dr. Srikumaran and Dr. Naveed Natanzi pointed out that the injection of other medications or blood products into the bursa or joint do not cause SIRVA like injuries.25
 NVIC’s public comments26 27 and December presentation28 to the ACCV outlined the reasons why syncope and SIRVA should not be removed from the VIT.29 NVIC drew attention to the federal reports that were critical of the lack of a consistency in how injuries were added to or removed from the VIT, and to the fact that the continued narrowing of the VIT only makes it more difficult for claimants to receive compensation for their vaccine related injuries.30 31
NVIC’s public comments26 27 and December presentation28 to the ACCV outlined the reasons why syncope and SIRVA should not be removed from the VIT.29 NVIC drew attention to the federal reports that were critical of the lack of a consistency in how injuries were added to or removed from the VIT, and to the fact that the continued narrowing of the VIT only makes it more difficult for claimants to receive compensation for their vaccine related injuries.30 31
NVIC’s December presentation also noted that research has continued to be published on syncope and SIRVA since the IOM’s report and should be reviewed prior to any change to the VIT relating to these injuries. The presentation also showed that there was no need to quell “frivolous” claims because historically the vast majority of injury claims have not been compensated. As December 1, 2020, DHHS reported that only 34 percent (7,705 out of 22,685) of all vaccine injury petitions filed with the VICP have been compensated.32
Steamrolling of the ACCV and Vaccine Injury Table
Despite the ACCV repeatedly requesting that DHHS present evidence as to why syncope and SIRVA should be removed from the VIT, DHHS has failed to present any evidence. As Mr. Milmoe, NVIC, and others have pointed out during ACCV meetings, this lack of participation on the part of DHHS is a stark departure from their actions when changes were made to VIT previously and typically included several presentations of evidence and a vote by the ACCV recommending a change, prior to issuance of an NPRM.33 34 35 36 37
Under federal law any changes to the VIT recommended by the ACCV are non-binding, due to their standing as an advisory committee. Therefore, now that the ACCV has made a recommendation to reject DHHS’ proposed removal of SIRVA and syncope from the VIT, that action is enough to allow the NPRM to move forward.
While DHHS has continued to refuse to present evidence to the ACCV as to why syncope and SIRVA should be removed from the VIT, NVIC, the vaccine injured, attorneys representing the vaccine injured, and health care professionals have presented evidence, opinions and concerns that overwhelmingly supported retaining syncope and SIRVA on the VIT.38 39 40
What Can You Do?

DHHS must respond to written public comments received on the NPRM. The deadline for written public comments on the proposed removal of syncope and SIRVA from the VIT is January 12, 2021. NVIC urges the public to oppose DHHS’ proposal to remove these injuries from the VIT for the following reasons:
- No evidence has been presented by DHHS justifying the removal of these injuries from the VIT;
- No data exists to support the DHHS position that the trust fund is running out of money. The latest data provided to the ACCV shows that there is ample money to furnish awards for these injuries as well as new injuries that may be added to the VIT;41 42
- VICP data does not support DHHS’ assertion that retaining these injuries will encourage frivolous petitions for compensation and add to DOJ’s caseload. In fact, the VICP routinely dismisses claims without merit and the removal of these injuries from the VIT will reduce the number of claims that can been resolved quickly, increase program costs, and provide no relief to caseloads;43 44
- The removal of these injuries from the VIT conflicts with the spirit and intent of the federal law governing the VICP.45
Read NVIC's referenced, public comment on this NPRM submitted to DHHS.
References:
1 HRSA. ACCV Webinar Recording – Mark 37.40 Mar. 6, 2020.
2 GPO.gov. 42 U.S.C. §§ 300aa-14. Vaccine Injury Table. 2016.
3 GPO.gov. 42 U.S.C. §300aa–19. Advisory Commission on Childhood Vaccines. 2016.
4 HRSA. Charter - Advisory Commission on Childhood Vaccines (ACCV). Jul. 20, 2018.
5 U.S. General Services Administration (GSA). Final - Federal Advisory Committee Rule 2001. Feb. 26, 2019.
6 Wadman, Meredith. United States wants to end most payouts for leading vaccination-related injury. Science Magazine. Apr. 20, 2020.
7 Fisher, BL. Williams, K. Wrangham, TK. Hendler, C. Letter to ACCV and ACCV DFO Re: Closed Door Discussions on Possible Removal of Syncope and Shoulder Injury Related to Vaccine Administration (SIRVA) from the Federal Vaccine Injury Table (VIT). Apr. 16, 2020.
8 Fisher, BL. William, K. Wrangham, TK. Hendler, C. Referenced Written Response & Request Relating to Proposal to Remove SIRVA and Syncope from the Federal Vaccine Injury Table. Apr. 16, 2020.
9 Federal Register. Advisory Commission on Childhood Vaccines - Notice. Apr. 27, 2020.
10 Institute of Medicine Committee. Adverse Events of Vaccines: Causality Assessment. Washington, DC: The National Academies Press 2012.
11 Institute of Medicine Committee. Adverse Events of Vaccines: Evidence and Causality - TABLE 12-1 Summary of Epidemiologic Assessments, Mechanistic Assessments, and Causality Conclusions for Injection-Related Adverse Events. Washington, DC: The National Academies Press 2012.
12 U.S. HRSA. ACCV Meeting Transcript. Mar. 8, 2012.
13 U.S. HRSA. ACCV Meeting Transcript. Mar. 8, 2012.
14 Federal Register. National Vaccine Injury Compensation Program: Revisions to the Vaccine Injury Table. Jan. 19, 2017.
15 U.S. HRSA. GUIDING PRINCIPLES FOR RECOMMENDING CHANGES TO THE VACCINE INJURY TABLE. Mar. 9, 2006.
16 HRSA. Division of Injury Compensation Programs Update to ACCV. Slide 11. Mar. 6, 2020.
17 HRSA. Division of Injury Compensation Programs Update to ACCV. Slide 11. Sept. 4, 2020.
18 HRSA. Division of Injury Compensation Programs Update to ACCV. Slide 11. Dec. 3, 2020.
19 HRSA. ACCV Minutes. Pg 2. Slide 11. Mar. 6, 2020.
20 HRSA. ACCV Recommendation - Implementation of the Draft National Vaccine Injury Compensation Program NPRM. May 20, 2020.
21 HRSA. ACCV Minutes. May 18, 2020.
22 HRSA. ACCV Minutes. May 18, 2020.
23 HRSA. ACCV Minutes. May 18, 2020.
24 HRSA. ACCV Minutes. May, 18,2020.
25 HRSA. ACCV Minutes. May, 18,2020.
26 Wrangham, T. NVIC Public Comment to ACCV. May 18, 2020.
27 Wrangham, T. NVIC Public Comment to ACCV. Dec. 3, 2020.
28 Wrangham, T. NVIC Presentation to ACCV – NPRM to Remove SIRVA and Syncope - Consumer Perspective. Dec. 3, 2020.
29 U.S. GAO. Vaccine Injury Trust Fund - Revenue Exceeds Current Need for Paying Claims. March 2020.
30 U.S. GAO. Vaccine Injury Compensation Program - Program Challenged to Settle Claims Quickly and Easily. December 1999.
31 U.S. GAO. Vaccine Injury Compensation - Most Claims Took Multiple Years and Many Were Settled through Negotiation. November 2014.
32 HRSA. Data & Statistics – Page 5. Dec. 1, 2020.
33 HRSA. Presentation to ACCV - Institute of Medicine (IOM) Report generated Proposals for Updates to the Vaccine Injury Table (VIT). Ryan, T. Dec. 9, 2011.
34 HRSA. Presentation to ACCV - Clarification on Proposed Changes to the Vaccine Injury Table. Houston, AM. Sep. 4, 2014.
35 HRSA. Presentation to ACCV - Prevention of SIRVA. Dalle-Tezze, T. Jun. 4, 2015.
36 HRSA. ACCV Minutes. Pg 5 – SIRVA. Jun. 3, 2016.
37 Federal Register. National Vaccine Injury Compensation Program: Revisions to the Vaccine Injury Table. Jan. 19, 2017.
38 HRSA. ACCV Draft Agenda. May 18, 2020.
39 HRSA. ACCV Draft Agenda. Sep. 4, 2020.
40 HRSA. ACCV Draft Agenda. Dec. 3, 2020.
41 HRSA. Division of Injury Compensation Programs Update to ACCV. Slide 10. Dec. 3, 2020.
42 U.S. GAO. Vaccine Injury Trust Fund - Revenue Exceeds Current Need for Paying Claims. March 2020.
43 HRSA. ACCV Minutes. Pg 2. Slide 11. Mar. 6, 2020.
44 HRSA. Division of Injury Compensation Programs Update to ACCV. Slide 8. Dec. 3, 2020.
45 GPO.gov. 42 U.S.C. §§ 300aa-14. Vaccine Injury Table. 2016.









Leave a comment
Your email address will not be published. Required fields are marked with an *