Read and report vaccine reactions, harassment and failures.
HPV: The Disease
Human papillomavirus (HPV) is the most commonly sexually transmitted infection in the U.S. There are more than 200 known types of HPV and about 75 percent of these types are considered not harmful. The most common high-risk cancer-causing HPV types are 16 and 18. There are six HPV-related cancers; cervical, oral (oropharyngeal), vaginal, penile, anal, and vulvar.
Most sexually active women and men become infected with HPV, with the majority of cases recovering from infection without symptoms or complications and more than 90 percent of cases recover within two years. Natural recovery provides HPV type specific antibodies within the individual’s body to help prevent future infections; however, the protection may not be life-long.
The American Cancer Society estimates in 2023 that out of an estimated total U.S. population of 334 million individuals a total of just over 30,000 (.009 percent) HPV-related cancer cases will occur and result in under 8,000 (.002 percent) deaths for HPV types targeted by Gardasil 9 vaccine. Learn more about HPV…
HPV Vaccine
While there are three FDA licensed HPV vaccines, only Gardasil 9 by Merck is currently available in the U.S. The CDC’s current recommendation is a two-dose series for ages 15 through 26 years, and those with immunocompromising conditions. Gardasil 9 by Merck was licensed to prevent HPV-related cervical, vulvar and anal cancers, genital warts, and precancerous lesions. According to the CDC, the effectiveness of HPV vaccination is still unknown and “it may take decades to see population-level impact” from HPV vaccine.
Though public health officials acknowledge there is minimal benefit in adult HPV vaccination, the vaccine is available to adults 26 through 45 years. For females over 21 years of age, HPV vaccination does not replace the need for routine Pap smear testing to detect abnormal cells that can cause cervical cancer especially considering that cervical cancer can be caused by HPV types not included in the vaccine. Learn more about HPV vaccine…
HPV Quick Facts
HPV
- Chronic infections are rare and occur mostly in women. Sometimes HPV infection does not clear from the body naturally and the infection becomes chronic. High risk factors for developing HPV-related cancers include: smoking, multiple sexual partners, long-term oral contraceptive use, multiple births, weakened immune system, co-infection with chlamydia or HIV, poor nutrition, heavy drinking and smoking, and chronic inflammation;
- After Pap smear tests that screen for cancerous conditions became a routine part of gynecological health care for American women in the 1960’s, U.S. cervical cancer cases dropped by 75 percent. Women are recommended to get regular Pap tests throughout their life whether or not they get HPV vaccinations; Continue reading quick facts…
HPV Vaccine
- Licensed in 2006 for 11-12 year old girls and young women, thousands of adverse reaction reports related to Gardasil were filed for: sudden collapse with unconsciousness within 24 hours, seizures, muscle pain and weakness, disabling fatigue, Guillain Barre Syndrome (GBS), facial paralysis brain inflammation, rheumatoid arthritis, lupus, blood clots, premature ovarian failure, optic neuritis, multiple sclerosis, strokes, heart and other serious health problems, including death were reported. Similar reports have been filed for the Gardasil 9 vaccine licensed in 2014 for use by females and males ages 9 to 45 years, even though the recommended number of doses was reduced from three to two.
- Using the MedAlerts search engine, as of June 30, 2023, the federal Vaccine Adverse Events Reporting System (VAERS) contains more than 74,229 reports of HPV vaccine reactions, hospitalizations, injuries and deaths and, includes 628 related deaths, 7,484 hospitalizations, and 3,578 disabling conditions. Over 55 percent of the reported serious adverse events occurred in children and teens under 17 years of age. Continue reading quick facts…
NVIC encourages you to become fully informed about HPV and the HPV vaccine by reading all sections in the Table of Contents below, which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
Analysis of Vaccine Adverse Events Reporting System Reports Part I
Adverse Reactions, Concerns and Implications
Human Papilloma Virus Vaccine Safety
Analysis of Vaccine Adverse Events Reporting System Reports Part I
Adverse Reactions, Concerns and Implications
by Vicky Debold, PhD, RN and Barbara Loe Fisher
Feb. 1, 2007
On June 8th 2006, the Food and Drug Administration (FDA) announced the approval of GARDASIL, and on June 29th the Advisory Committee on Immunizations Practices (ACIP) voted to recommend adding GARDASIL human papilloma virus vaccine to the Centers for Disease Control's national childhood recommended immunization schedule. On July 14th the first report of a serious reaction to the vaccine was filed with the federal Vaccine Adverse Event Reporting System (VAERS).
A 16-year-old Illinois girl was vaccinated July 7th and 13 days later developed symptoms eventually diagnosed as Guillian-Barre Syndrome. A 14-year-old girl in the District of Columbia was vaccinated on July 11th and complained of severe pain immediately following the injection, fell off the examining table and experienced a 10 to 15 second fainting spell ending up in the emergency room with a headache and speech problems. The report of this reaction, the first in the nation, was filed on July 14th, 15 days after the ACIP vote.
Six months later, 82 reports of GARDASIL reactions have been submitted to VAERS on behalf of at least 84 young girls and 2 boys.[1] Reaction reports have come in from 21 states and the District of Columbia.[2] Reactions were reported for children and young adults ranging in age from 11 to 27. Of the reports indicating what day the vaccine was given and the reaction occurred, 63 percent stated that the reaction occurred the same day the vaccine was given. All but three of the reports were for reactions that occurred within one week of vaccination.
This document is divided into three sections. The first section describes reaction reports for a number of reported adverse events: neurological symptoms including syncopal episodes and seizures, arthralgia and joint pain, Guillian-Barre Syndrome, and other immunological reactions. The second section addresses concerns related to vaccinating individuals already infected with HPV. The last section discusses issues that need to be addressed by government regulators and the manufacturer and considerations for clinicians and consumers.
Reported Adverse Events
Presumably, the reactions described below occurred after the first dose of GARDASIL. GARDASIL is given in a three-dose series. None of the reports stated that the children and adults experiencing problems had previously been vaccinated with GARDASIL.
Syncopal Episodes and Seizures. One-quarter of all reports filed after GARDASIL vaccination were for neurologic adverse events including loss of consciousness, syncope, syncopal events and seizures. An additional five reports included symptoms of dizziness and feeling faint.
Syncope is defined as a temporary suspension of consciousness due to generalized cerebral ischemia (inadequate blood flow and lack of oxygen). The reports of syncopal episodes and their descriptions are remarkable. A physician from Washington State reported that in one morning, three patients experienced syncopal episodes. On August 8th another physician's office reported that two patients experienced syncopal episodes on the same day.
Although these reports did not detail what happened to the individuals experiencing these syncopal episodes, other reports did. The 14-year-old DC girl mentioned earlier experienced a syncopal episode combined with amblyopia (poor vision in one eye), abnormal speech, vomiting, and headache. Also experiencing vision problems, a 17-year-old New York girl reported feeling dizzy and her vision went "black for a few seconds" and she turned pale and lips turned purple and she also had fever and chills. Similar to the DC girl, on July 18th immediately after being vaccinated, a 22-year-old Kentucky woman experienced slurred speech accompanied by pallor and shock. On August 29th, two hours after being vaccinated, a 15-year-old New York girl who had a history of asthma and was on four asthma medications experienced difficulty swallowing prompting a visit to the emergency room. On August 17th, 15 minutes after being vaccinated, a 14-year-old Pennsylvania girl passed out in the car on the way home.
Most of the reports do not describe what happened as a result of the syncopal episode but a few do. One 11-year-old Florida girl fell from the examining table and two Washington girls fell - a 16-year-old girl fell and hit her head on a carpeted concrete surface and a 14-year-old girl fell down and broke her nose.
Whether the 22 girls who experienced syncopal episodes actually experienced atonic seizures cannot be determined from these reports. Four girls, however, displayed observable seizure activity. The 11-year-old Florida girl who fell from the table also displayed "tonic posturing." Tonic posturing is a type of seizure where sustained contraction of muscles in the legs and arms occurs and consciousness is impaired. The 16-year-old Washington girl who fell and hit her head on the floor lost consciousness for one minute and displayed tonic posturing of her right hand. Additionally, a 15-year-old girl from Virginia was described as having "a mild seizure." In California, a 13-year-old girl was walking down the hall after her vaccination, fell and had a 15-second tonic/clonic seizure. Tonic/clonic seizures are also known as "grand mal" seizures.
Additionally, there were reports of dyskinesia (difficulty or distortion in performing voluntary movements) and hypokinesia (slow or diminished movement of the body musculature) both of which have neurological implications.
Arthralgia, Joint Pain and Fever. Arthralgia is defined as pain in the joints. Concerns about arthritis were raised during the GARDASIL clinical trials. Reports of arthralgia in one or more joints accompanied by fever were noted in five instances from four young girls and women in Wisconsin, Texas and New York, and one 18-year-old New York male.
Guillain-Barre Syndrome. Reports state that two recently vaccinated 16-year-old girls - one from Illinois and the other from Mississippi - were diagnosed with Guillian-Barre Syndrome (GBS) following vaccination with GARDASIL. In both cases, the onset of symptoms occurred 13 days after vaccination. According to the National Institute for Neurological Disorders and Stroke:
GBS is a serious disorder in which the body's immune system attacks part of the peripheral nervous system. The first symptoms of this disorder include varying degrees of weakness or tingling sensations in the legs. In many instances, the weakness and abnormal sensations spread to the arms and upper body. These symptoms can increase in intensity until certain muscles cannot be used at all and, when severe, the patient is almost totally paralyzed. … Vaccinations can trigger onset of GBS.[3]
The Illinois girl described earlier was vaccinated on July 7th and symptoms were evident by July 20th. The girl also experienced gait abnormalities (trouble walking properly), asthenia (weakness without loss of strength), paresthesia (burning, prickling, tingling or numbness sensation usually felt in the hands, arms, feet and legs), and hyperkinesia (abnormal increase in muscle movement). The Mississippi girl was vaccinated on July 31st and by August 13th she had increasing numbness and tingling in her feet and hands and was subsequently evaluated by a neurologist and diagnosed with GBS. The current health status of these girls is not known.
In both of these cases, the girls were also vaccinated with Aventis Pasteur's Menactra, a vaccine for meningococcal infections. Menactra has previously been associated with Guillain-Barre Syndrome, and the FDA and others have issued alerts.
Other Adverse Reactions. Additionally, a number of other reactions to GARDASIL are noted in VAERS reports and they include: urticaria (hives); pruritus (itching); macular and papular rashes; blisters and vesicles near the injection site; swollen arms; lymphadenopathy (swollen lymph nodes); red, hot swollen knots at injection site; burning, stabbing, severe and radiating pain at the injection site and in the affected limb during and after injection; nausea and vomiting; infections and skin ulcers, and other allergic reactions.
Other Considerations
GARDASIL is marketed as a "cervical cancer vaccine" and intended to prevent infection with specific HPVs - common viruses among sexually active women. It isn't clear what benefits or potential harms could arise from vaccinating sexually active women who have already contracted HPV. Of the 86 reaction reports filed with VAERS so far, 12 reports were generated by young women 18 and older who were taking hormonal contraceptives and presumably sexually active.
With respect to concerns related to vaccinating women with known HPV infections, adverse reaction reports were filed on behalf of a 17-year-old Texas girl who was already diagnosed with HPV and genital warts. Similarly, the 22 year-old Kentucky woman who experienced slurred speech following vaccination already had an abnormal pap smear with evidence of cervical dysplasia.
Implications
The early reports of potential safety problems with GARDASIL raise concerns and questions that need to be addressed by government regulators, manufacturers and prescribing physicians. Specifically, the following concerns need to be addressed:
- Syncope, seizures and Guillian-Barre Syndrome have now been reported with hours to a week after GARDASIL vaccination. GARDASIL manufacturer, Merck, should add these serious adverse events to the product manufacturer insert.
- Considering that over 20 girls have experienced syncopal episodes sometimes combined with seizures and serious injuries, physicians should consider only giving GARDASIL when the patient is safely laying down on the examining table. Because there seems to be syncopal reactions up until 15 minutes after vaccination, patients should be asked to lie down for 15 minutes after receipt of GARDASIL.
- The information provided by Merck indicates that it is safe to administer GARDASIL with Hepatitis B vaccine. The prescribing information states, "Results for clinical studies indicate that GARDASIL may be administered concomitantly (at a separate injection site) with hepatitis B vaccine (recombinant). Co-administration of GARDASIL with other vaccines has not been studied." [4] Due to the small number of girls aged 9 to 15 who appear to have been evaluated for GARDASIL safety in Merck clinical trials (fewer than 2,000) and lack of publicly available information about how many of these girls were given GARDASIL and hepatitis B vaccine simultaneously, the safety of administering GARDASIL and hepatitis B vaccine to all pre-adolescent girls is uncertain.[5]
- Aside from Hepatitis B, Merck does not state that it is safe to simultaneously administer GARDASIL with any other vaccine. Considering that there are ongoing evaluations of a reported association between Menactra (meningococcal vaccine) and Guillain-Barre Syndrome, and Merck does not explicitly indicate that it is safe to administer to administer GARDASIL and Menactra simultaneously, consumers and clinicians should question whether administering both GARDASIL and Menactra at the same time is safe.
- Similarly, adverse reactions were reported when GARDASIL was administered with eight other vaccines: Hepatitis A, MNQ (?), MEN (Menactra), TD (Tetanus and Diptheria Toxoids), DPP (Diptheria/Pertussis/Polio), PNC Prevnar (Heptavalent pneumococcal conjugate), DTaP (Diphtheria And Tetanus Toxoids and Acellular Pertussis Vaccine), and TDAP (Tetanus, Diptheria and Pertussis). Because Merck does not state that it is safe to administer simultaneously GARDASIL with any vaccine other than Hepatitis B, consumers and clinicians should question whether co-administration of GARDASIL and other vaccines is safe.
- Most, if not all, of the reactions reported to VAERS were in response to the first of the three doses of GARDASIL. The Centers for Disease Control (CDC) Vaccine Information Sheet (VIS) developed for HPV vaccine states that severe reactions include "any unusual condition, such as a high fever or behavior changes. Signs of a serious allergic reaction can include difficulty breathing, hoarseness or wheezing, hives, paleness, weakness, a fast heart beat or dizziness." [6] The CDC also states that "anyone who has ever had a life-threatening allergic reaction to yeast, to any other component of HPV vaccine, or to a previous dose of HPV vaccine should not get the vaccine." Which of the reactions reported to VAERS constitute a "life-threatening allergic reaction" and which, if any, of the children and young adults who experienced reactions should receive additional doses of vaccine? At the October 2006 ACIP meeting, CDC staff stated that only "three serious reports were reported to VAERS after HPV vaccination in females 14 and 16 years of age. One of these patients had vasovagal syncope and was hospitalized overnight for observation." [7]CDC's summary of the first 76 VAERS reports suggests that CDC doesn't regard the remaining reports as "serious." CDC needs to clarify which of the reactions reported to VAERS constitute contraindications to further vaccination with GARDASIL and make this information available to the public and to prescribing physicians.
- What were the short and longer-term outcomes for the individuals who experienced the reactions reported to VAERS? Is there information available that would help to predict the characteristics that predispose one to be at greatest risk of experiencing a serious reaction?
- The CDC's Vaccine Information Sheet indicates that allergy to yeast is a reason to avoid taking GARDASIL. Merck notes that contraindications to the vaccine include "hypersensitivity to the active substances or to any of the excipients of the vaccine. Individuals who develop symptoms indicative of hypersensitivity after receiving a dose of GARDASIL should not receive further doses of GARDASIL." The prescribing information provided by Merck does not specifically note that yeast allergy is a contraindication to taking GARDASIL. Government regulators and the manufacturer need to address the discrepancy between these documents and clarify the issues related to yeast allergy and make this information readily available to the public and prescribing physicians.
- Additionally, Merck notes that vaccine ingredients include 225 mcg of aluminum (as amorphous aluminum hydroxyphosphate sulfate adjuvant), 0.78 mg of L-histidine, 50 mcg of polysorbate 80, and 35 mcg of sodium borate. These ingredients are not listed on the CDC's VIS sheet. The public needs this information so that they can identify whether they have "hypersensitivities" to any of the ingredients and whether they are at risk of experiencing a serious allergic reaction. Hypersensitivities and known allergic reactions are critical pieces of information that need to be communicated to prescribing physicians in order to make the safest possible vaccination decisions.
Government regulators including the CDC and FDA, in combination with Merck, should address the above safety concerns as soon as possible. Medical groups advocating use of GARDASIL should effectively communicate to physicians and patients the potential risks of using GARDASIL along with precautions to improve the safety of patient care.
For more information about HPV infection, GARDASIL vaccine and VAERS adverse event reports pertaining to GARDASIL, go to www.NVIC.org
[1] National Vaccine Information Center. VAERS reports related to HPV4 vaccine http://www.medalerts.org/vaersdb/findfield.php?PAGENO=2&PERPAGE=10&VAX=HPV4 (Accessed January 10, 2007).
[2] States reporting adverse reactions include: Arkansas (1), Arizona (3), California (14), Colorado (1), District of Columbia (1), Florida (4), Georgia (1), Illinois (2), Kansas (1), Kentucky (3), Massachusetts (4), Maine (1), Missouri (1), North Carolina (4), New Jersey (2), New York (7), Pennsylvania (4), Rhode Island (3), Tennessee (1), Texas (9), Virginia (1) and Washington (4). State of residence was not identified in at least 14 cases.
[3] National Institute of Neurological Disorders and Stroke. Guillain-Barre Fact Sheet http://www.ninds.nih.gov/disorders/gbs/detail_gbs.htm. Accessed January 31, 2007.
[4 ] Merck and Co., Inc. GARDASIL. www.merck.com/product/usa/pi_circulars/g/GARDASIL/GARDASIL_pi.pdf (Accessed January 31, 2007).
[5] Food and Drug Administration. Product Approval Information – Licensing Action: GARDASIL Questions and Answers. http://www.fda.gov/cber/products/hpvmer060806qa.htm. Study 016: Studies in Younger Age Groups, page 19; Study 018: Safety and immune response younger children, page 20.
[6]Centers for Disease Control, National Immunization Program, HPV Vaccine Information Sheet www.cdc.gov/nip/publications/VIS/vis-hpv.pdf (Accessed January 31, 2007.)
[7]Centers for Disease Contol, National Immunization Program, ACIP Meetings October 2006 http://www.cdc.gov/nip/ACIP/minutes.htm (Accessed January 31, 2007.)
What is HPV?
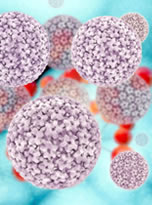
Human papillomaviruses are double-stranded DNA viruses found in the squamous epithelial cells on the surface of the skin and also the mucous membranes of the body. There are more than 200 known types of human papilloma viruses (HPVs) and most are not harmful. In the majority of cases, the human immune system clears HPV infections without symptoms or complications.
More than 90 percent of those who become infected naturally clear the infection from the body within two years. Antibodies to the HPV type causing the infection remain in the body to help prevent future infections but the protection may not be life-long.
Low Risk HPV Types - About 75 percent of HPVs have been associated with non-cancerous warts (papillomas) on the hands, chest, arms and feet, such as low-risk (wart-causing) HPV types 6 and 11. Low-risk HPV types associated with genital warts differ from high-risk HPV types that can be associated with development of cancer after years of chronic infection.
High Risk HPV Types - About 40 HPV types have been found in the body’s mucosal membranes, such as the mucosal surfaces of the cervix, vagina, vulva, anus, penis, mouth and throat, including the most common high-risk HPV types 16 and 18. High-risk HPV types are associated with cancer of the cervix and five other genital and oral cancers affecting women and men if the HPV infection does not clear and becomes a chronic infection. High-risk HPV types currently include types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 69, 73 and 82. The National Cancer Institute states that most HPV-related cancers are cause by two HPV types, HPV-16 and HPV-18.
IMPORTANT NOTE: NVIC encourages you to become fully informed about HPV and the HPV vaccine by reading all sections in the Table of Contents , which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
Survey of HPV Vaccine (Gardasil) Cost
NVIC Pivate Pediatrician Office Survey
Northern Virginia Area HPV Vaccine (GARDASIL) Costs
January 2007
In January, 2007 NVIC conducted a small survey of private pediatrician offices in the Washington, D.C. area. Four pediatric practices in the Northern Virginia area were contacted to determine the total cost of the HPV three-dose series, administration fees and office visit charges.
The cost for GARDASIL vaccine and administration fees ranged from $420 to $825 for a three-dose series plus additional charges for each office visit to receive the shot. The additional charges for the three office visits related to administration of the vaccine varied depending upon if the patient
(1) is a current patient;
(2) is a new patient;
(3) the vaccine is administered by a physician;
(4) the vaccine is administered by a nurse;
(5) the visit is for a well-child and preventive care;
(6) the visit is for sick child care.
Based on this information, total costs for the series of three GARDASIL vaccinations, related administration fees and office visit charges are estimated to range from $500 to $900 or more.
Pediatric Practice #1
Contacted 1/31/07
GARDASIL Vaccine and Administration Fee: $420 ($140 per shot)
Office Visit Charge: $150 to $185 for new patient or no charge to $88 for current patient.
Three Shot Series: Minimum $420 plus office visit charges.
Pediatric Practice #2
Contacted 1/31/07
GARDASIL Vaccine and Administration Fee: $555 ($185 per shot)
Office Visit Charge: $110-205 for new patient, less for current patient.
Three Shot Series: Minimum $555 plus office visit charges.
Pedatric Practice #3
Contacted 1/7/07
GARDASIL Vaccine and administration fee: $525 ($175 per shot)
Office visit charge: $35 to $83
Three Shot Series: Minimum $630
Pedatric Practice #4
Contacted 1/31/07
GARDASIL Vaccine and Administration Fee: $825 ($275 per shot)
Office Visit charge: $82 for new patient, less for current patient.
Three Shot Series: Minimum $825 plus office visit charges.
Is HPV contagious?

HPV is contagious, and more than 40 HPV types can be transmitted through sexual contact with an infected person. These 40 HPV types are found in the mucosal membranes of the body and transmitted through vaginal, oral or anal sex.1 Most of these HPV infections are asymptomatic and result in no clinical disease.2 Per the CDC, “More than 90 percent of new HPV infections, including those caused by high-risk HPV types, clear or become undetectable within 2 years, and clearance usually occurs in the first 6 months after infection.”3 In very rare cases, genital HPV types can be spread from mother to infant.4
Most HPV types - 75 percent - are skin (cutaneous) infections and are spread by skin-to-skin contact and are not sexually transmitted. Cutaneous HPV types can be found on the arms, hands, feet and legs5 and are associated with common warts.6
IMPORTANT NOTE: NVIC encourages you to become fully informed about HPV and the HPV vaccine by reading all sections in the Table of Contents , which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
What is the history of HPV in America and other countries?
The first recorded research linking a sexually transmitted disease to cervical cancer dates back to an 1842 study by Rigoni-Stern, an Italian physician. After reviewing 80 years of female death certificates, he noted that cervical cancer deaths almost exclusively occurred in married or widowed women, and prostitutes. Early cervical cancer research focused on the Herpes Simplex II virus but this theory was disproven in 1984.
In 1965, the first published HPV study characterized its DNA. Prior to 1965, papillomavirus studies focused on rabbit papillomavirus and its association to cancer. During the 1970’s, more than one type of HPV was recognized and by 1972, work was underway to evaluate an association between HPV and cervical cancer.
In 1982, several studies associating HPV type 6 with genital warts, but not cervical cancer, were published. In 1983, HPV type 16 was identified in cervical cancer cells. A year later, HPV type 18 was linked to cervical cancer.
HPV infections are endemic around the world and public health officials believe that it is the most common sexually transmitted infection in the US with an estimated 14 million new infections occurring annually. Public health officials estimate that 79 million people in the US are infected with HPV, a common infection in adolescents and young adults.
Prevalence in U.S. Women – A 2007 study of female HPV prevalence reported that 26.8 percent of 14 to 59-year olds were infected but, among 20 to 24-year olds, 44.8 percent were infected. Low-risk HPV types 6 and 11 and high-risk HPV types 16 and 18 detected in 3.4 percent of females evaluated in the study. Researchers concluded that:
- Although HPV infection is common, studies suggest approximately 90 percent of infections clear within 2 years,
- HPV is common among U.S. females but the prevalence of the HPV types contained in the vaccine is relatively low, and
- High-risk HPV types are detected in 99 percent of cervical cancers and worldwide, approximately 70 percent of cervical cancers are due to HPV types 16 and 18.
In the early 1960’s, after Pap smear tests to screen for cancerous conditions became a routine part of women’s gynecological health care in the US, cases of HPV-associated cervical cancer dropped by 75 percent. By 2006, the year Gardasil came to market, because of Pap test screening, new annual cervical cancer cases declined to about 9,700 and deaths to about 3,700 within a US population of more than 300 million.
Prevalence in U.S. Men - A 2006 review of 40 HPV prevalence studies found that, among U.S. males, prevalence rates ranged from 1.3 to 72.9 percent. The researchers concluded that:
- Data on the frequency of acquisition and the duration of HPV infection in men are limited,
- HPV prevalence rates in men vary widely and have been reported to be as high as what has been reported in women, and
- Screening for HPV infection in men is not routinely recommended because infection is very common, no FDA-approved screening test is available and HPV infection does not increase the risk of disease or cancer in men or their sex partners.
IMPORTANT NOTE: NVIC encourages you to become fully informed about HPV and the HPV vaccine by reading all sections in the Table of Contents , which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
Human Papilloma Virus Vaccine Safety
Analysis of Vaccine Adverse Events Reporting System Reports Part II
Human Papilloma Virus Vaccine Safety
Analysis of Vaccine Adverse Events Reporting System Reports Part II
by Vicky Debold, PhD, RN and Barbara Loe Fisher
February 21, 2007
This document is a follow-up to the February 1, 2007 report detailing adverse events, concerns and clinical implications related to use of GARDASIL based on submissions made to the federal Vaccine Adverse Event Reporting System (VAERS). This report covers all reactions submitted in 2006 and supplements the first NVIC analysis of reported adverse events - NVIC Analysis of VAERS Reports on GARDASIL - which can be found here. Readers are encouraged to review the previous report. A number of clinical and patient safety concerns and potential problems arising from co-administration of GARDASIL with other vaccines not evaluated by the manufacturer were discussed in the previous report.
Also, readers are referred to the VAERS website for cautions related to use of VAERS data.[1] The information in this report does not prove a cause and effect relationship between any of the reactions and administration of GARDASIL. This document only summarizes the information in VAERS as it relates to use of GARDASIL, either alone or in combination with other vaccines.
[2]
As of mid-February 2007, there were 430 case reports associated with administration of the GARDASIL vaccine. After the duplicate case records were removed (about 10 percent of files), 385 unique case records remained. The data from the 385 case reports are used for this analysis unless stated otherwise.
Since GARDASIL was licensed on June 8, 2006 and added to the recommended American vaccination schedule on June 29, 2006, the number of reports per month filed with VAERS has steadily increased. Two reports were filed in July, 12 in August, 65 in September, 79 in October, 108 in November and 119 in December. Between June 8 and December 31st, an average of 1.87 [3] reports per day have been filed. In December, 3.97 reports per day were filed.
As previously reported, the earliest adverse event report date following vaccine licensure and recommendations was July 14, 2006 for a girl vaccinated July 7, 2006. The earliest reported adverse event overall was for a woman vaccinated on September 1, 2003 during a clinical trial. The most recent report included in this analysis was submitted December 28, 2006.
Reports were submitted to VAERS at various dates throughout each month but tended to be grouped around four dates. In particular, 247 reports (64 percent of all reports) were submitted on September 19th (44 reports), October 17th (45 reports), November 16th (108 reports) and December 18th (87 reports).
Patient Demographics
In 98 case reports (25.4 percent) age was not identified. Patient age, where reported, ranged from 1 to 57 years. Among the case reports where age was identified, 111 reports (38.7 percent) were submitted for children 16 and under. The distribution by age of these children is as follows: 1.0/1.5 years (2 cases)[4], 9 (1 case), 11 (9 cases), 12 (10 cases), 13 (14 cases), 14 (25 cases), 15 (27 cases) and 16 (23 cases).
In 14 reports, the gender of the patient was not identified. In all but four of the remaining cases, the patients were females. The males for whom case reports were submitted were 1.0, 1.5, 12 and 18-years-old.
As of the end of December, case reports have been submitted to VAERS from 43 states and the District of Columbia. Two months earlier, reports had been submitted from 20 states.
Description Of Reported Reactions
In the case reports submitted to VAERS, five of the reactions were described as "life-threatening," six were "disabling," and 210 (54.5 percent) had "not recovered" as of the date data were provided by VAERS. Hospitalization was reported in 12 cases and two-thirds sought additional care in an emergency room or doctor's office (see Use of Health Services section).
A number of symptoms were reported, with varying levels of severity.[5] The most frequently reported reactions were: pain of various types (89), syncope (55) and dizziness (41), fever/pyrexia (41), paresthesia and hypoaestesia (32), rash (33) and pruritis/itching (31), vasodilation (19), headache (19), and vomiting (16). The following sections focus on three reactions: syncope and dizziness, paresthesia, and Guillain-Barre Syndrome.
Descriptions of the symptoms presented in the following condition-specific sections includes verbatim passages taken from VAERS case reports. No attempt was made to correct spelling or modify what was reported. Symptom description passages are often truncated mid-sentence in the VAERS database.
The time of reaction onset was noted in 192 reports (72 percent). Among the reports that noted when the reaction occurred, 60 percent (115 reports) indicated a reaction on the day the vaccine was given. An additional 21 percent (41 reports) stated that the reaction occurred the day after the vaccine was given. Reports indicated that 94 percent (181 reports) occurred within one week of vaccination.
Syncope
Syncope is defined as a temporary suspension of consciousness due to generalized cerebral ischemia (inadequate blood flow and lack of oxygen).
At the end of December 2006, there were 62 case reports stating that a patient had experienced a syncopal episode suggesting that 14.4 percent of case records involve syncope and/or fainting (62/430).[6] Among children 9 to 16 years of age, 12.2 percent of case reports (14/115) documented a syncopal episode. In comparison, only 5.8 percent of children 9 to 16 years of age who received the Tdap (tetanus-diptheria-pertussis) vaccine experienced a syncopal episode.[7]
When syncope is considered along with reports of dizziness (41 cases), approximately 25 percent of all VAERS case reports are involved. Although most syncope reports indicate that it occurred immediately after vaccine administration, some do not. Despite the impression that syncopal episodes are commonplace and harmless, some of the episodes appear to be atypical and worthy of attention. Specifically of concern are the reactions that occur well after the vaccine was given and, in some cases, are combined with seizures and injuries because the patients were either in a sitting or standing position when the syncopal event occurred, fell, and suffered injuries including fractures as a result. For example:
- Case record - 269192 - (14 y/o) - Information has been received from a physician concerning a 14 year old female who on an unspecified date was vaccinated with Gardasil (yeast). It was reported that the patient was sitting on a bench and when the personnel left the room, the patient apparently fainted and ended up falling off bench. It was reported that it was unsure if the patient had broken her nose but there was blood. At the time of this report, the outcome of the events were unknown. The physician feels that the patient "might be anore [report truncated]
- Case record - 260144 - (13 y/o) - Patient received Hep A in right arm. Then received HPV in left arm. C/O pains, numbness. Started walking down hall fainted and had tonic/clonic movement for 15 sec. Symptoms: Dyskinesia, hypertonia, hypotonia, pain, paresthesia, syncope
- Case record - 265305 - (21 y/o) - According to my daughter, the following happened: 1. Shot was given between 1100-1115 am 2. Proceed to wait a few minutes with doctor in room and then went to check out 3. Between 1120-1125 am...she passed out in doctors office. 4. Day 2-4: tightness in muscles especially around lower legs. Also tigtness in arms, but not as bad as legs. 5. Hard time walking after first waking up in morning and gradually loosens up after she starts moving around. 6. I suggested to her to take a Tylenol to help with m [report truncated]. Symptoms: Gait abnorm, hypertonia, syncope.
- Case record - 262242 - (14 y/o) - Vasovagal syncope shortly after receiving hep A and Gardasil vaccine, fell, hit nose on a drawer, loss of consciousness, sent to ER in transport broke nose. Symptoms: Bone fract spontan, injury accid, syncope.
- Case record - 264959 - (14 y/o) - Administr4ed Vaqta, then Gardasil, immediately after Gardasil child became unresponsive, stiff, pale about 30 sex. Child lost bladder control, pulse 46-55 after event, child alert and aware after events. Symptoms: Arrhythmia, bradycardia, hypertonia, incontin urin, pallor, stupor, syncope.
NVIC again raises questions about the advisability of allowing children who are believed to be susceptible to syncopal episodes to be vaccinated in a sitting position, left unattended, and not required to lie down for at least 5 to 15 minutes following vaccination.
Paresthesia [8]
Paresthesia (paraesthesia) is an abnormal sensation of the skin, such as numbness, tingling, pricking, burning, or creeping on the skin that has no objective cause. Hypesthesia (hypoaesthesia) is the name of the condition that occurs when the skin loses some of its sensitivity to pain or touch. Both have a number of causes and are symptoms of a number of both minor and serious diseases and conditions.
As of the end of December 2006, there were 34 case reports stating that patients had experienced either paresthesia, paraesthesia or hypoaesthesia episodes. Thus, 7.9 percent of case records involve reports of paresthesia-type symptoms (34/430).[9] Among children 9 to 16 years of age, 7.0 percent of case reports (8/115) documented a paresthesia episode. In comparison, only 1.6 percent of children 9 to 16 years of age who received the Tdap vaccine (tetanus-diptheria-pertussis) experienced paresthesia symptoms.
- Case record - 264761 - (18 y/o) - Information has been received from a physician concerning his "around 18" year old daughter who was vaccinated IM with a 0.5 ml dose of HPV rL1 6 11 16 18 VLP vaccine (yeast). Concomitant therapy included a dose of diphtheria toxoid (+) pertussis acellular 3-component vaccine (+) tetanus toxoid (BOOSTRIX). Subsequently, the patient experienced numbness in her arms and lower extremities. Unspecified medical attention was sought. On an unspecified date, the patient recovered. No product quality complaint was [report truncated]. Symptoms: Hypesthesia.
- Case record - 264950 - (57 y/o) - 09/19/2006 MMR and HBV adm 1, 10/03/2006 swollen glands, numbness and tingling of arm and legs bilat. Pt called hotline and states she was told this was from the MMR. 10/15/06 chest pain. Pt wonders if this is related to immunizations. Symptoms: Lymphadenopathy, chest pain, paresthesia.
- Case record - 267171 - (13 y/o) - INfluenza vaccine and Human Papilloma VIrus vaccines given November 10 about 11:45 AM. Patient woke up with numbness on right side of face on November 11. Presented to Emergency Room on November 12 with Bells Palsy of right side of face. Unable to move right side of face. Unable to close right eye. Previously healthy, no symptoms on day of vaccine administration. Symptoms: Paralysis, facial paralysis, paresthesia.
- Case record - 268459 - (14 y/o) - With in five minutes of administration complaint of headache, then felt faint. I had my daughter sit down in the waiting room with her head down before we walked out. Not thinking this had anything to do with the vaccine. We walked out the door and she began have difficlty walking, weaving, I asked her if she was okay and she could only respond yes. I took her arm and tried to turn her back to the Doctor's office and she collapsed on the ground. Though, she remainded conscious her eyes were glazed and [record truncated]. Symptoms: Cold sweat, difficulty in walking, disorientation, dizziness, dyskinesia, headache, hyperventilation, pallor, paraesthesia, tinnitus, tremor
Guillain-Barre Syndrome
According to the National Institute for Neurological Disorders and Stroke:
As of the end of December 2006, there were five case reports that identified Guillain-Barre Syndrome in GARDASIL recipients. The first two of the case reports were described in NVIC's first report. As previously stated, both girls had also received a meningococcal vaccine at the same time that they received GARDASIL.[10]
The three new case reports involve two 13-year-old girls, from Arkansas and Washington, and a 16-year-old from Ohio. All three of these girls received only GARDASIL.
- Case report - 265839 - (16 y/o) - Information has been received from a physician concerning a 16 year old female who on an unspecified date was vaccinated with the first dose of HPV vaccine. subsequently the pt developed lower extremity weakness and was hospitalized for two days with Guillain Barre. The pt received treatment with gamma globulin during her hospitalization. At the time of this report, the pt was back to school and was 99% recovered having only some area of numbness to her lower extremities. The physician did not believe that [report truncated]. Symptoms were: asthenia, Guillain-Barre Syndrome, and paresthesia.
- Case report - 268143 - (13 y/o) - Pt admitted to hospital on 12/1/06 with chief complaint of ascending weakness bilaterally, upper and lower extremities. Neuro consult diagnosed Guillain-Barre syndrome. Pt received the Gardasil vaccine on 11/22/06. Resident MD asked pharmacist to write up possible ADR of Guillain-Barre from this vaccine. Symptoms: CSF test abnormal, Guillain-Barre Syndrome, muscular weakness
- Case report - 269328 - (13 y/o) - Information has been received from a physician concerning a 13 year old female who on 22-Nov-2006 was vaccinated with HPV rL1 6 11 16 18 VLP vaccine (yeast). A few days later she had cold symptoms and was prescribed azithromycin (ZITHROMAX). A few days after that the patient developed hives and was prescribed amoxicillin. A few days later the patient was hospitalized and diagnosed on 01-Dec-2006 with Guillian-Barre Syndrome. The patient sought unspecified medical attention. At the time of the report, it was [reported truncated] Symptoms: Guillain-Barre Syndrome, urticaria (hives).
Human Papilloma Virus Infections
There were several case reports of infection with human papilloma virus and cervical dysplasia and genital warts. For example, case reports 260907, 267457, and 269228 describe infections with genital warts, and 267410, 267480, 269248 reported infection with HPV; and 263204, 267415, 268483, 269192, 269202, 269213 report abnormal pap smears and in some cases, cervical dysplasia. A few of the case reports note that procedures to address dysplasia were planned including colposcopy and loop electrosurgical excision procedures.
In most cases, it was not documented whether patients were infected prior to vaccination and whether an exacerbation of disease was experienced following vaccination. Two exceptions include:
- Case report - 267457 - (age not reported) - Information has been received from a midwife registered nurse concerning a female with genital warts who "within the last few weeks" was vaccinated with HPV rL1 6 11 16 18 VLP vaccine (yeast). Concomitant therapy included imiquimod (Aldara). Subsequently "within the last few weeks" the patient experienced "swelling in genital warts". At the time of the report the patient was recovering. Unspecified medical attention was sought. Other information has been requested. Symptoms: Edema
- Case report - 267418 - (22 y/o) - Information has been received spontaneously from a gynecologist concerning a 22 year old female patient who had participated in an HPV study. On 29-SEP-2006 the patient tested positive for high risk HPV. The patient's outcome was unknown. Upon internal request, the patient was unblinded on 25-OCT-2006, she received her first dose of HPV on 06-FEB-2003, her second dose in March 2003, and her third dose in September 2003. She showed strong conversion to all 4 vaccine types. At baseline the patient had been se…[report truncated]. Diagnostic lab data - 07-DEC-2004: "ASC-H". 04-APR-2005 colposcopy biopsy negative for lesion, discrepancy noted - biopsy was of less severity than the Papanicolaou (PAP) test result and repeat colposcopy, PAP, and endocervical curettage (ECC) (among other choic…[report truncated]. Symptoms: No drug effect
Co-administration of Vaccines
The manufacturer's insert only evaluated co-administration of GARDASIL with Hepatitis B vaccine. The case reports submitted to VAERS indicate that GARDASIL has been administered simultaneously with 18 additional vaccines. As categorized by VAERS, the following vaccines were given along with GARDASIL: DPP, DTAP, DTOX, FLU, FLUN, HEPA, HEPAB, HEP, IPV, MEN, MMR, MNQ, TDAP, TTOX, VARCEL, HIB, MMRV, PNC, and TD. The case reports show that 42 patients (13.9 percent) received at least one other vaccine along with GARDASIL. Additionally, 13 patients received three vaccines, three received four vaccines, and one, the 1-year-old infant, received five vaccines. In only two case reports was Hepatitis B the additional vaccine simultaneously administered. Among those who received two or more vaccines, 68.3 percent were 16 or younger. Of those 16 or younger for whom an adverse event report was submitted, 25.2 percent had received two or more vaccines.
Use Of Health Services Among Patients Reporting A Reaction
The case reports indicate that in 253 instances (66 percent), additional ambulatory services were used. It is not possible to determine how many total additional visits were made or to distinguish how many of the additional visits were made to an emergency room rather than a physician's office. Of those requiring additional visits, 57 (22.5 percent) were made by children 15 and under. Among all children 15 and under, 64.8 percent required an additional emergency room or doctor's office visit.
Case reports indicate that 12 patients ranging in age from 13 to 23 were admitted to the hospital and stayed up to five days. Table 1 reports the age and state of residence of the patient. It also shows the number of days following vaccination, use of vaccines other than GARDASIL, symptoms, narrative description and use of tests as noted in the VAERS database.
Concerns
NVIC previously raised concerns about the seriousness of some of the reported reactions. Syncopal episodes combined with seizures and injury, as well as certain types of paresthesia, weakness, paralysis and Guillain-Barre Syndrome are serious health events. In the interest of protecting the public's health, action is needed on the part of all stakeholders.
Doctors, nurses and parents should promptly report all reactions following vaccination to VAERS. Failure to submit reports of all reactions to VAERS weakens efforts to monitor and improve the safety and effectiveness of the national vaccination program.
Doctors, nurses and parents should note that a substantial number of children are experiencing dizziness and syncopal episodes after vaccination and some are sustaining injuries when they lose consciousness and fall. This is a clear patient safety issue and children should be protected from potential and unnecessary injury whenever possible. They should be vaccinated laying down and required to lie down for at least 5 to 15 minutes after vaccination. They should not be left unattended.
The manufacturer's product insert should be updated to include explicit precautions related to use of GARDASIL in combination with vaccines not evaluated during clinical trials. Nearly 25 percent of children were vaccinated with one or more of 18 vaccines not evaluated by the manufacturer. There are no evidence-based guidelines that support use of such a protocol. NVIC is also concerned that the numbers of children vaccinated with Hepatitis B along with GARDASIL during the clinical trials were insufficient to establish safety. Until the adverse reactions reported to VAERS can be evaluated fully, doctors and nurses should not administer other vaccines at the same time GARDASIL is given.
The manufacturer's product insert should also be updated to include the serious reactions that have been reported to VAERS.
[1] Information about the VAERS reporting system can be found here http://vaers.hhs.gov. The government has issued the following warning to people using VAERS data: When evaluating data from VAERS, it is important to note that for any reported event, no cause and effect relationship has been established. VAERS is interested in all potential associations between vaccines and adverse events. Therefore, VAERS collects data on any adverse event following vaccination, be it coincidental or truly caused by a vaccine. The report of an adverse event to VAERS is not documentation that a vaccine caused the event.
[2] Unless otherwise noted, our report uses the data for adverse events reported July 1, 2006 to November 30, 2006 and retrieved on two dates in January and February 2007. For the adverse events reported December 1 through December 31, 2006, our report uses data retrieved mid-February 2007. As of February 18, 2007 some of the data in the VAERS database – reported symptoms, in particular -- have been modified. See footnote related to paresthesia.
[3] Between recommendation date (6/29/07) and December 31, 2007, 2.07 reports per day have been filed with VAERS. Per day reports by month: July .06, August .39, September 2.63, October 2.55, November 3.60, and December 3.97.
[4] In two cases, GARDASIL was unintentionally given to two male infants.
[5] Symptoms reported are as follows (in alphabetical order): acne (3), abasia (2), abdominal pain (3), abnormal behaviour (1), abortion (1), agitation (3), albuminuria (1), allerg react (3), amblyopia (3), amenorrhea (1), angioedema (1), anorexia (1), anxiety (2), applicat site react (2), arrhythmia (3), arthralgia (7), asthenia (14), asthma (1), bacterial infection (1), blood glucose increased (1), blood potassium decreased (2), blood pressure decreased (1), blood pressure increased (1), blood urine present (1), body temperature decreased (1), bone fract spontan (1), bradycardia (2), breast pain (1), cellulitis (2), chest pain (3), chills (7), cold sweat (1), colitis (1), condition aggravated (2), confusion (2), conjunctivitis (1), contusion (2), convulsion (5), convulsion grand mal (1), cough inc (1), csf test abnormal (1), culture urine positive (1), cyanosis (1), decreased appetite (2), dehydration (3), depersonal (1), depression (1), diarrhea (2), difficulty in walking (1), diplopia (1), discomfort (1), disorientation (1), dizziness (41), dry eye (1), dyskinesia (2), dysphagia (1), dyspnea/ dyspnoea (7), dysuria (1), ecchymosis (1), edema/oedema (9), edema face (8), edema inject site (4), edema/oedema periph (8), edema tongue (1), emotion labil (1), epistaxis (1), erythema (2), esr inc (1), euphoric mood (1), eyes gaze upward (1), fall (1), fatigue (1), feeling abnormal (1), fever (36), flu synd (4), flushing (1), fracture (1), gait abnorm/disturbance (3), guillain barre synd (5), haemorrhage (1), headache (19), hem (1), hepatitis (2), herpes simplex (2), high-pitched crying (1), hostility (1), hyperhidrosis (5), hyperkinesia (3), hypersensitivity (1), hypertonia (9), hyperventil (2), hypesthesia (5), hypoaesthesia (4), hypokinesia (4), hypotens (1), hypotonia (1), hypoxia (1), hysn inject site (5), incontin urin (1), incorrect route of drug administration (1), infect (2), infect fung (2), infect viral (2), inflam inject site (1), influenza like illness (5), inject site react (13), injection site anaesthesia (1), injection site erythema (4), injection site induration (1), injection site mass (9), injection site oedema (2), injection site pain (5), injection site rash (2), injection site swelling (1), injury accid (5), insomnia (2), joint stiffness (1), lab test abnorm (7), lactation fem (1), laryngismus (3), loss of consciousness (6), lymphadenopathy (10), malaise (12), med error (9), menorrhagia (1), menstrual disorder (1), menstruation irregular (1), migraine (2), movement dis (1), mucous mem dis (1), muscular weakness (2), myalgia (13), mydriasis (1), myositis (1), nausea (30), neopl skin (1), nervousness (1), no drug effect (1), nodule skin (1), pain (38), pain abdo (5), pain back (1), pain chest (2), pain inject site (37), pain pelvic (1), pallor (9), paralysis (1), paralysis facial (3), paresthesia/paraesthesia (28), pelvic pain (1), pharyngitis (2), photosensitivity (1), platelet count decreased (1), post vac synd (2), pregn unintend (1), pregnancy test false positive (1), pregnancy test negative (1), pruritus (31), pruritus genital (1), pyrexia (5), rash (26). rash erythematous (1), rash mac pap (5), rash macular (1), rash vesic bull (5), react aggrav (2) react uneval (4), rhinitis (1), rhinorrhoea (1), scan brain (1), screaming synd (1), sensation of heaviness (3), shock (1), similar reaction on previous exposure to drug (1), skin discolor (2), skin disorder (1), skin papilloma (1), smear cervix abnormal (2), spasm general (2), speech dis (2), stomatitis ulcer (1), stupor (2), suicide attempt (1), sweat (5), swelling (2), syncope (55), taste pervers (1), thinking abnorm (1), tinnitus (2), tremor (3), twitch (2), ulcer skin (2), unintended pregnancy (1), urticaria (23), vaccination complication (1), vaginitis (2), vasc dis periph (1), vasodilat (19), vertigo (1), viral infection (2), vision abnorm (1), vision blurred (2), visual disturbance (1), vomit (16), wheezing (1), white blood cell count increased (1), white blood cells urine positive (1).
[6] Computations in this section used VAERS data that contained duplicate entries. NVIC found duplicate records in the VAERS database, including among case reports of syncope. After correcting for duplicate records, 14.3 percent of patients (55/385) experienced a syncopal episode.
[7] At the same office visit, three children also received Hepatitis A vaccine and one received chickenpox vaccine.
[8] When the VAERS database was checked on two different dates in February 2007, in a number of cases the person reporting the adverse event to VAERS (e.g., a doctor, nurse, or parent) included the symptom paresthesia. However, in the current version of the VAERS database, a number of these earlier reports have been modified without explanation. Either the symptom was removed entirely or re-spelled as “paraesthesia” or more frequently, reclassified as hypoaesthesia. For the this analysis, the three terms were grouped.
[9] Computations in this section used uncorrected VAERS data that contained duplicate entries. Using data corrected for duplicate entries, the rate for paresthesia-type symptoms was 8.3 percent (32/385).
[10] National Institute of Neurological Disorders and Stroke. Guillain-Barre Fact Sheet http://www.ninds.nih.gov/disorders/gbs/detail_gbs.htm. Accessed January 31, 2007.
[11] Data in Table 1 are taken verbatim from the VAERS database. The narratives within case reports are frequently truncated mid-sentence. Additionally, data for age, date of vaccination and interval between vaccination and onset of symptoms is sometimes missing.Can HPV cause injury and death?

HPV infection usually causes no symptoms and most women and men clear the infection within one to two years. Antibodies to the HPV type that caused the infection remain in the body to help prevent future infections of that HPV type but the duration of protective immunity is unknown.
Most, But Not All, HPV Infections Resolve Spontaneously - Sometimes an HPV infection does not clear from the body and thus, becomes a chronic infection. After many years of undetected chronic HPV infection, cervical, and other genital or oral cancers can develop and cause disability or death. The CDC states that, “Although the incidence of infection is high, most infections resolve spontaneously. A small proportion of infected persons become persistently infected; persistent infection is the most important risk factor for the development of cervical cancer.”
Persistent HPV infection associated with development of cervical cancer is clinically manifested in women by cervical intraepithelial neoplasia (CIN), which are referred to as “pre-cancerous” lesions. Low-grade CIN (CIN 1) may spontaneously resolve when infection clears from the body or it may progress to CIN 2 or CIN 3, which may lead to cervical cancer, if the pre-cancerous lesions are left undetected and untreated for years.
The American Cancer Society states “Cervical cancer is most frequently diagnosed in women between the ages of 35 and 44 with the average age at diagnosis being 50 . It rarely develops in women younger than 20. Many older women do not realize that the risk of developing cervical cancer is still present as they age. More than 20% of cases of cervical cancer are found in women over 65. However, these cancers rarely occur in women who have been getting regular tests to screen for cervical cancer before they were 65.”
Women Need Pap Test Screening - Whether women have gotten HPV vaccinations or not, routine Pap test screening is recommended for all women to detect high grade CINs and receive prompt treatment in order to prevent cervical cancer from developing.
Six HPV-Related Cancers - In addition to cervical cancer, there are five other cancers associated with chronic HPV infection: mouth and throat (oropharyngeal), vaginal, penile, anal and vulvar. In 2023, the American Cancer Society estimates that in the U.S. (population of over 334 million):
- About 13,960 cases of invasive cervical cancer will be diagnosed and result in 4,310 deaths.
- About 54,540 cases of oral cavity or oropharyngeal cancer will be diagnosed and result in 11,580 deaths.
- About 8,470 cases of vaginal cancer will be diagnosed and result in 1,740 deaths.
- About 2,050 cases of penile cancer will be diagnosed and result in 470 deaths.
- About 9,760 cases (6,580 in women and 3,180 in men) of anal cancer will be diagnosed and result in 1,870 deaths (860 in women and 1,010 in men).
- About 6,470 cases vulva cancers will be diagnosed and result in 1,670 deaths.
The CDC states, “About 10% of women with high-risk HPV on their cervix will develop long-lasting HPV infections that put them at risk for cervical cancer. Similarly, when high-risk HPV lingers and infects the cells of the vulva, vagina, penis, anus, or the oropharynx (back of the throat, including the base of the tongue and tonsils), it can cause cell changes called precancers. These may eventually develop into cancer if they're not found and removed in time. These cancers are much less common than cervical cancer. Much less is known about how many people with HPV will develop cancer in these areas.”
Low Number of HPV-Related Cancer Deaths – Each year, six HPV associated cancers cause 21,640 deaths, less than three percent of the 602,350 annual U.S. cancer deaths.
IMPORTANT NOTE: NVIC encourages you to become fully informed about HPV and the HPV vaccine by reading all sections in the Table of Contents , which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
Who is at highest risk for getting HPV?

Non-cutaneous HPVs are typically transmitted sexually. Over the course of their lifetime, more than 80 percent of women and 90 percent of men will become infected with at least one HPV type.
Risk factors for acquiring HPV are directly related to sexual behavior of the individual and their partners. Epidemiologic studies are inconclusive as to whether other risk factors including genetics, age of sexual initiation, number of pregnancies, lack of circumcision of male partner, and tobacco use increase a person’s susceptibility to HPV infection.
IMPORTANT NOTE: NVIC encourages you to become fully informed about HPV and the HPV vaccine by reading all sections in the Table of Contents , which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
Gardasil Rally Statement
Barbara Loe Fisher, Co-Founder and President March 8, 2007, Washington, D.C.
STATEMENT
Barbara Loe Fisher, Co-Founder and President
National Vaccine Information Center
March 8, 2007, Washington, D.C. Rally sponsored by
Parents and Citizens Committee to Stop Medical Experimentation in D.C.
Just 15 days after the Centers for Disease Control recommended that all 11 and 12 year old girls in America get three doses of Merck’s HPV vaccine, GARDASIL, the very first GARDASIL reaction report was filed with the federal Vaccine Adverse Event Reporting system. The report described a 14 year old District of Columbia girl, who was injected with GARDASIL on July 11, 2006 and complained of severe pain at the injection site, lost consciousness, fell off the examining table, regained consciousness and experienced tingling, numbness, pain and twitching in her hands and feet, headache and blurry vision. She vomited in the parking lot, lost her speech and was sent to the Emergency Room, where she continued to exhibit difficulty speaking upon neurological examination. This first GARDASIL reaction report, which included collapse and loss of sensation in the hands and feet along with other neurological signs, was a prophetic warning. In the seven months following licensure of GARDASIL, many of the more than 600 vaccine reaction reports involve similar kinds of serious neurological symptoms among young girls and women.
The National Vaccine Information Center joins with the Parents and Citizens Committee to Stop Medical Experimentation in D.C. in calling for public hearings and citizen testimony about whether 11 year old girls living in the nation’s Capitol should be required by law to get three doses of HPV vaccine in order to attend sixth grade. This call for more citizen involvement in new vaccine laws is taking place in every state where HPV vaccine mandates are being aggressively pursued by drug company lobbyists and legislators trying to force young girls to use HPV vaccine without the voluntary, informed consent of parents.
Since 1982 the National Vaccine Information Center has defended the right of parents to have full information about the benefits and risks of vaccines and be allowed to exercise informed consent to vaccination. We oppose HPV vaccine mandates for three reasons.
First, there is a serious absence of scientific evidence that Merck’s GARDASIL is safe to give to young girls entering puberty, who are biologically different from older women. Vaccine mandates target 11 year old girls but Merck only studied the safety of GARDASIL in a few hundred eleven year old girls and followed them up for a few years. Already, there have been more than 600 reports made to the federal Vaccine Adverse Event Reporting System documenting that young girls are collapsing and suffering seizures, loss of sensation in the hands and feet, vision and speech problems, Guillain Barre syndrome, facial paralysis, and other serious neurological symptoms. Merck also admits in its product insert that GARDASIL was not studied for the ability to affect female fertility, cause other kinds of cancer or be toxic to the genes.
Second, there is little or no scientific evidence that Merck’s GARDASIL will actually protect 11 year old girls from getting cervical cancer. The vaccine only contains two high risk HPV types out of 15 types associated with cervical cancer. Officials at the Food and Drug Administration and Centers for Disease Control have expressed concern that the 13 other high risk HPV types could replace the two types in GARDASIL so that HPV associated cervical cancer may not, in fact, be eliminated with widespread use of the vaccine. Young girls may think they can skip routine pap smears because the vaccine protects them from getting cervical cancer when that may not be true at all.
Third, more than 90 percent of all girls and boys, who become infected with HPV, asymptomatically clear the infection from the body. It takes many years of chronic infection for undiagnosed pre-cancerous cervical lesions to progress to cervical cancer, usually because the women have not had routine cancer -detecting pap smears. Cervical cancer has dropped by more than 74 percent in the US since pap smears became part of routine health care for women and today less than one percent of all cancer deaths and newly diagnosed cancers are due to cervical cancer in America.
The more than $4 billion dollars it will take to vaccinate every 11 year old girl in Washington, D.C. and every other state could be better spent on research to find out why so many highly vaccinated children in Washington, D.C. and other public school systems around the nation are chronically ill and disabled, with 1 in 150 autistic, one in 6 learning delayed and millions more asthmatic and diabetic.
In conclusion, HPV vaccine mandates are unnecessary, expensive and potentially dangerous because so little is known about the long term health risks and effectiveness of Merck’s GARDASIL vaccine. Elementary schools should be centers for learning and not centers for forced use of potentially unsafe and ineffective vaccines. The District of Columbia should hold open public hearings and allow parents to present testimony about why they want to become fully informed about the benefits and risks of HPV vaccine and be allowed to make a voluntary vaccination decision for their daughters.
Who is at highest risk for suffering complications from HPV?

Risk factors for developing cancer, including HPV associated cancers, differ depending upon the cancer type, personal health, and lifestyle choices including those related to smoking, drinking and diet. That said, having one or more risk factors (or no risk factors) does not necessarily determine whether or not cancer will develop.
At highest risk of suffering HPV infection complications are the less than ten percent of women and men who do not naturally clear high-risk HPV infections and therefore, become chronically infected.
According to the National Cancer Institute, factors that can increase the risk of developing cancer following chronic infection with high-risk HPV types include:
- Smoking
- Having a weakened immune system
- Diagnosis with an aggressive HPV type
The American Cancer Society lists the following risk factors for developing cervical cancer (after years of undiagnosed and untreated chronic infection with high risk HPV types):
- Smoking
- Immunosuppression
- Chlamydia Infection
- Poor diet and obesity
- Long term oral contraceptive use
- Multiple full-term pregnancies (three or more)
- Young age (under 18 years old) at the first full term pregnancy
- Poverty/lack of access to Pap tests
- Women whose mothers took the hormonal drug Diethystilbestrol (DES) during pregnancy (1940-1971)
- Family history of cervical cancer
Oral cavity and oropharyngeal cancers:
- Tobacco use, including chewing tobacco
- Alcohol use
- Heavy drinking and smoking
- HPV infection
- Gender (men at greater risk)
- Age (over age 55)
- UV Light
- Poor nutrition
- Genetic syndromes
Anal cancer:
- HPV infection
- Other genital cancers (increased risk for women)
- HIV infection
- Multiple sexual partners
- Smoking
- Lowered immunity
- Race and gender
Vaginal and vulvar cancers:
- HPV infection
- Cervical precancer or cervical cancer
- Weakened immune system
- Smoking
- Chronic vulvar itching or burning
Penile cancer:
- HPV infection
- Poor hygiene combined with lack of circumcision
- Smoking and other tobacco use
- UV light treatment of psoriasis
- Age (over age 55)
- AIDS
IMPORTANT NOTE: NVIC encourages you to become fully informed about HPV and the HPV vaccine by reading all sections in the Table of Contents , which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
Gardasil & Menactra Adverse Event Reports to the Vaccine Adverse Events Reporting System (VAERS)
An Analysis by the National Vaccine Information Center of
Gardasil & Menactra Adverse Event Reports to the
Vaccine Adverse Events Reporting System (VAERS)
February 2009
The centralized federal Vaccine Adverse Events Reporting System (VAERS) was created under the National Childhood Vaccine Injury Act of 1986 and became operational in 1990. Although there was a mandate in the 1986 law for vaccine providers to report serious health problems, hospitalizations, injuries and deaths to VAERS, there were no sanctions for failure to report.
It is estimated that only between 1 and 10 percent of all adverse health outcomes which occur following vaccination are reported to VAERS. The following information was produced from analyzing VAERS data published by CDC and FDA and made available on the www.NVIC.org website through the MedAlerts searchable VAERS database at www.MedAlerts.org
(through November 30, 2008)
Menactra (meningococcal) vaccine was licensed by the FDA on January 14, 2005 for children and adults aged 11 to 55 years. One dose is recommended by the CDC for boys and girls 11-12 years old and many high schools and colleges are requiring it for attendance. Menactra is routinely administered by pediatricians and family practice physicians to grade school, high school and college students. Between 1,000 and 2,600 cases of meningococcal disease occurs in the U.S. every year and Menactra contains four of the most common strains of meningococcal.
Gardasil (Human Papillomavirus Quadrivalent) vaccine was licensed by the FDA on June 8, 2006 for girls and women aged 9 to 26 years. Three doses are recommended by the CDC for universal use in girls 11-12 years old and Gardasil is routinely administered by pediatricians, obstetrician/gynecologists and family practice physicians to grade school, high school and college students. Chronic human papillamovirus infection is associated with the development of cervical cancer and Gardasil contains four HPV types associated with this sexually transmitted infection.
What follows is a comparison of some of the more serious Gardasil and Menactra adverse event reports submitted to VAERS.
|
SUMMARY STATISTICS |
Gardasil (HPV) |
Comment on Gardasil Reports |
Menactra |
Comment on Menactra Reports |
|
Total Reports |
10,151 |
|
4,436 |
|
|
Life Threatening |
152 |
|
57 |
(18 with HPV) |
|
Emergency Room |
5,021 |
|
1,667 |
|
|
Hospitalization |
458 |
|
268 |
|
|
Did Not Recover |
2,017 |
|
393 |
|
|
Disabled |
261 |
|
29 |
(13 with HPV) |
|
Died |
29 |
(25 HPV alone) |
6 |
(none with HPV) |
|
|
||||
|
SYMPTOM STATISTICS |
|
|
|
|
|
Alopecia |
51 |
(41 HPV alone) |
12 |
(8 with HPV) |
|
Arrhythmia |
18 |
(14 HPV alone) |
4 |
(1 with HPV) |
|
Arthralgia |
276 |
|
119 |
|
|
Arthritis |
68 |
(57 HPV alone) |
18 |
(6 with HPV) |
|
Blindness |
36 |
(31 HPV alone) |
10 |
(2 with HPV) |
|
Blood clot |
23 |
(all HPV alone) |
0 |
|
|
Cardiac arrest |
9 |
(all HPV alone) |
2 |
(none with HPV) |
|
Chest pain |
123 |
|
63 |
(16 with HPV) |
|
Collapse |
44 |
(29 HPV alone) |
18 |
(7 with HPV) |
|
Deafness |
17 |
(11 HPV alone) |
5 |
(4 with HPV) |
|
Demyelination |
12 |
(10 HPV alone) |
10 |
(1 with HPV) |
|
Dizziness |
1,320 |
|
589 |
|
|
Encephalomyelitis |
8 |
(5 HPV alone) |
9 |
(1 with HPV) |
|
Fainting |
278 |
|
41 |
(28 with HPV) |
|
Hair Loss |
36 |
(30 HPV alone) |
8 |
(5 with HPV) |
|
Hemorrhage |
9 |
(7 HPV alone) |
2 |
(2 with HPV) |
|
Joint pain |
126 |
|
37 |
(7 with HPV) |
|
Lupus |
28 |
(27 HPV alone) |
6 |
(1 with HPV) |
|
Migraine |
159 |
|
36 |
(9 with HPV) |
|
Multiple sclerosis |
16 |
(12 HPV alone) |
7 |
(2 with HPV) |
|
Numbness |
351 |
|
149 |
|
|
Pain |
2,422 |
|
1,320 |
|
|
Paralysis |
70 |
(54 HPV alone) |
26 |
(6 with HPV) |
|
Rash |
963 |
|
473 |
|
|
Rheumatoid arthritis |
31 |
(25 HPV alone) |
6 |
(4 with HPV) |
|
Seizures |
544 |
|
158 |
(73 with HPV) |
|
Stroke |
16 |
(all HPV alone) |
1 |
(none with HPV) |
|
Syncope |
1,643 |
|
427 |
|
|
Thrombosis |
34 |
(all HPV alone) |
1 |
(none with HPV) |
|
Tingling |
278 |
|
132 |
|
|
Vasculitis |
11 |
(all HPV alone) |
2 |
(none with HPV) |
|
Rechallenge |
275 |
|
8 |
(7 with HPV) |
Red Flags:
VAERS is a sentinel reporting system, designed to raise “red flags” for unusual numbers of serious adverse events following receipt of a newly licensed vaccine. In 2005, the FDA responsibly issued a public advisory to physicians and parents after five (5) cases of Guillain Barre Syndrome (GBS) following receipt of Menactra by 17 and 18 year old girls occurred. A rough comparison of Gardasil and Menactra adverse event reports to VAERS through November 30, 2008 reveals that:
- Compared to Menactra, receipt of Gardasil is associated with at least twice as many Emergency Room visit reports; 4 times more Death reports; 5 times more “Did Not Recover” reports; and 7 times more “Disabled” reports.
- Compared to Menactra, receipt of Gardasil is associated with all of the reports of Blood Clots. All 23 reports of Blood Clots following Gardasil occurred when Gardasil was given alone without any other vaccines.
- Compared to Menactra, receipt of Gardasil is associated with at least 4 times as many Cardiac Arrest reports. All 9 reports of Cardiac Arrest following Gardasil occurred when Gardasil was given alone without any other vaccines.
- Compared to Menactra, receipt of Gardasil is associated with at least 6 times as many Fainting reports and at least 3 times as many Syncope reports.
- Compared to Menactra, receipt of Gardasil is associated with at least 4 times as many Lupus reports. 27 reports of Lupus following Gardasil occurred when Gardasil was given alone
- Compared to Menactra, receipt of Gardasil is associated with at least 15 times as many Stroke reports. 16 reports of Stroke following Gardasil occurred when Gardasil was given alone.
- Compared to Menactra, receipt of Gardasil is associated with at least 3 times as many Syncope reports.
- Compared to Menactra, receipt of Gardasil is associated with at least 33 times as many Thrombosis reports. 34 reports of Thrombosis following Gardasil occurred when Gardasil was given alone.
- Compared to Menactra, receipt of Gardasil is associated with at least 5 times as many Vasculitis reports. 11 reports of Vasculitis following Gardasil occurred when Gardasiil was given alone.
- Compared to Menactra, receipt of Gardasil is associated with at least 30 times as many Rechallenge reports, which involve a worsening of symptoms experienced after previous receipt of Gardasil.
Conclusion:
In 2007, the National Vaccine Information Center issued three analyses of VAERS reports following receipt of Gardasil. In these reports, we warned that Gardasil appeared to be associated with an unusually high number of reports of atypical collapse (fainting and syncope) and other symptoms of brain and immune system dysfunction.
A rough analysis of adverse events reported to VAERS following receipt of Gardasil and/or Menactra vaccines through November 30, 2008 indicate that Gardasil is involved in a much higher number of serious adverse health events than Menactra.
Although Gardasil is given in a three- shot series and only one dose of Menactra is given, Menactra is given to both boys and girls while Gardasil is given only to girls. It is unusual for there to be such a big discrepancy between two vaccines used in similar populations involving serious and relatively rare life threatening adverse events and autoimmune disorders such as death, blood clots, cardiac arrest, lupus, thrombosis, stroke, and vasculitis.
Fainting, which has been attributed by doctors and health officials as “fear” of needles in teenage girls is reported six times as often (and Syncope is reported three times as often) after receipt of Gardasil than Menactra even though Menactra is also given to girls in the same age group.
In pre-licensure clinical trials, Gardasil was only tested in fewer than 1200 girls 16 years and younger. Through November 30, 2008, in girls 16 or younger, there were reports of 9 deaths; 3 blood clots; 4 cardiac arrests; 9 cases of lupus; 6 strokes; and 2 cases of vasculitis developing after receipt of Gardasil.
This VAERS analysis gives compelling evidence for the need for action to be taken by the FDA, CDC and Congress:
- the FDA should further investigate reports of serious health problems and deaths following Gardasil vaccination; review the accuracy of information about adverse events contained in product manufacturer inserts; and inform physicians and parents about all serious health problems that have been reported to VAERS after Gardasil vaccination;
- the CDC should re-investigate VAERS reports of serious health problems and deaths after Gardasil vaccination; consider the need to withdraw the recommendation that all girls between the ages of 9 and 26 should receive Gardasil vaccine; and issue a warning that, when a serious adverse event occurs after Gardasil vaccination, no further Gardasil shots should be given;
- physicians in the fields of pediatrics and obstetrics/gynecology should fully inform patients and parents about all reported Gardasil adverse events and refrain from re-vaccinating those who experience serious health problems following Gardasil vaccination;
- Merck and the NIH should separately conduct studies into the biological mechanisms for Gardasil vaccine injury and death and define them for physicians and the public so: (a) biological high risk factors can be identified to facilitate informed medical decisionmaking ; (b) pathological profiles can be developed to confirm Gardasil- induced brain and immune system dysfunction and death; (c) healing therapies to moderate Gardasil- induced brain and immune system dysfunction can be developed; and (d) Merck can improve the safety of Gardasil;
- Congress should investigate the fast- tracking of Gardasil vaccine without adequate long- term safety studies in American pre-adolescent and teenage girls between ages 9 and16 and the safety and effectiveness of Gardasil vaccine in all age groups.
Vaccines which are licensed and recommended by the government for universal use by children and young adults, should adhere to the highest standards with regard to proof of safety and efficacy. In October 2008, the government issued a report maintaining that receipt of Gardasil is not associated with more serious health problems, hospitalizations, injuries and deaths among young girls and women than are experienced by those young girls and women who do not receive Gardasil. This analysis comparing adverse events reports to VAERS following receipt of Gardasil and Menactra appears to contradict that assertion.
Immediate action should be taken now by federal health agencies to protect recipients of Gardasil from injury and death.
Can HPV be prevented and are there treatment options?

HPV infection prevention options focus on refraining from or limiting sexual activity in terms of numbers of partners and consistent use of condoms during all types of sexual activity including vaginal, anal and oral. There are no recommended treatments for HPV infection, which usually clears naturally, but there are options for treating genital warts and pre-cancerous lesions that develop after chronic infection.
Treatment Options: Anogenital warts and precancerous lesions are currently the only two conditions that warrant treatment.
Treatment options for anogenital warts include use of anti-tumor medications, cryotherapy or surgical removal. Without treatment, anogenital warts may resolve spontaneously, remain the same, or may increase in number and size. HPV testing of genital warts is not recommended because the results will not affect treatment.
Treatment options for precancerous cervical cells often identified subsequent to a Pap test, include the following options: cryotherapy (a process to freeze and destroy the cells), laser therapy (use of light beam to remove or kill the cells), loop electrosurgical excision procedure (LEEP) (electric current is passed through a wired loop and used as a blade to excise abnormal cells) and conization (cone-shaped tissue sample is cut away by either a laser, a knife, or by use of the LEEP procedure).
Prevention Options:
HPV Vaccination
The FDA has approved three vaccines: Cervarix, Gardasil, and Gardasil 9 for the prevention of HPV infection. Currently, only Gardasil 9, a 9-valent recombinant vaccine targeting HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58, is available for use in the U.S.
According to the CDC, in addition to recommending the HPV vaccine, prevention options include:
- Abstaining from sexual activity (vaginal, anal, and oral).
- Using physical barriers, such as condoms, to reduce the risk of HPV transmission.
- Engaging in a monogamous relationship with an uninfected partner.
IMPORTANT NOTE: NVIC encourages you to become fully informed about HPV and the HPV vaccine by reading all sections in the Table of Contents , which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
What is HPV vaccine?

There are three FDA licensed HPV vaccines, however only one, Gardasil 9, approved in 2014 and manufactured by Merck, is currently available in the United States. Initially, HPV vaccines were given as a series of three shots over 6 months to protect against HPV infection and the health problems that ongoing HPV infection can cause. In 2016 the CDC recommended a two-dose series with second dose administered between 6 to 12 months from the first dose. Three doses of the vaccine are recommended if the vaccine is initially administered at age 15 through 26 years, and for those with immunocompromising conditions. Adults 26 through 45 years may choose to receive HPV vaccine, however, public health officials have acknowledged that the benefit to vaccination in this population is minimal. Below is information on the HPV vaccines licensed in the U.S.
- Gardasil 9: FDA approved Gardasil 9 for use in 2014. The safety of Gardasil 9 was studied in clinical trials with more than 15,000 participants before it was licensed and continues to be monitored. According to Merck’s product insert, Gardasil 9 protects against HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58.
- Gardasil: FDA approved Gardasil for use in 2006. The safety of Gardasil was studied in clinical trials with more than 29,000 participants before it was licensed. According to Merck’s product insert, Gardasil protects against HPV types 6, 11, 16, and 18.
- Cervarix: FDA approved Cervarix for use in 2009. The safety of Cervarix was studied in clinical trials with more than 30,000 participants before it was licensed. According to GlaxoSmithKine’s product insert, Cervarix protects women and girls against HPV types 16 and 18.
Vaccines, like any medicine, can have side effects. Some people who get HPV vaccine have no side effects at all. Some people report having very mild side effects, like a sore arm from the shot. According to manufacturer product inserts, the most common side effects reported are usually mild and include:
- Pain, redness, or swelling in the arm where the shot was given
- Fever
- Headache or feeling tired
- Nausea
- Muscle or joint pain
According to the Gardasil 9 product insert, brief fainting spells and related symptoms (such as jerking movements) can happen after any medical procedure, including vaccination. Sitting or lying down for about 15 minutes after a vaccination can help prevent fainting and injuries caused by falls.
On very rare occasions, severe (anaphylactic) allergic reactions can occur after vaccination. People with severe allergies to any component of a vaccine should not receive that vaccine.
Gardasil 9 is approved for use in girls and women ages 9 through 45 to prevent genital warts caused by Human Papillomavirus (HPV) types 6 and 11 and for the prevention of cervical, anal, vaginal, and vulvar cancers associated with HPV types 16, 18, 31, 33, 45, 52, and 58. Per the manufacturer’s product insert, Gardasil 9 also targets the following dysplastic and precancerous lesions associated with HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58:
- Cervical intraepithelial neoplasia (CIN) grade 1
- Cervical adenocarcinoma in situ (AIS) and cervical intraepithelial neoplasia (CIN) grade 2/3
- Anal intraepithelial neoplasia (AIN) grades 1, 2, and 3
- Vaginal intraepithelial neoplasia (VIN) grade 2 and 3
- Vulvar intraepithelial neoplasia (VIN) grade 2 and 3
Gardasil 9 is also approved for use in boys and men ages 9 through 45 to prevent genital warts caused by HPV types 6 and 11 and for the prevention of anal cancer associated with HPV types 16, 18, 31, 33, 45, 52, and 58. Per the manufacturer’s product insert, Gardasil 9 targets anal intraepithelial dysplasia (AIN) grades 1, 2, and 3 associated with HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58.
According to Merck’s product insert, Gardasil 9, Human Papillomavirus 9-valent Vaccine, Recombinant, is a non-infectious recombinant 9-valent vaccine approved by the FDA for intramuscular administration. This vaccine is produced from the purified virus-like particles (VLPs) of the major capsid (L1) protein of HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58. The L1 proteins are formed by separate fermentations using recombinant Saccharomyces cerevisiae and self-assembled into virus-like particles. The fermentation process necessitates the growth of S. cerevisiae on a chemically-defined fermentation media which include carbohydrates, mineral salts, vitamins, and amino acids. The virus-like particles are freed from the yeast cells by cell disruption and purified by a series of physical and chemical methods. The purified virus-like particles are adsorbed on preformed Amorphous Aluminum Hydroxyphosphate Sulfate (AAHS), an aluminum containing adjuvant. The 9-valent Human Papillomavirus VLP vaccine is a sterile liquid suspension that is prepared by combining the adsorbed virus-like particles of each HPV type as well as additional amounts of the aluminum-containing adjuvant, AAHS, and the final purification buffer.
Merck’s Gardasil 9 product inserts states that each 0.5-mL dose contains approximately 30 mcg of HPV Type 6 L1 protein, 40 mcg of HPV Type 11 L1 protein, 60 mcg of HPV Type 16 L1 protein, 40 mcg of HPV Type 18 L1 protein, 20 mcg of HPV Type 31 L1 protein, 20 mcg of HPV Type 33 L1 protein, 20 mcg of HPV Type 45 L1 protein, 20 mcg of HPV Type 52 L1 protein, and 20 mcg of HPV Type 58 L1 protein.
Each 0.5-mL dose of the vaccine also contains approximately 500 mcg of aluminum (in the form of Amorphous Aluminum Hydroxyphosphate Sulfate), 9.56 mg of sodium chloride, 0.78 mg of L-histidine, 50 mcg of polysorbate 80, 35 mcg of sodium borate, <7 mcg yeast protein, and water for injection.
The majority of pre-licensure clinical trials compared Gardasil 9 to Merck’s original Gardasil vaccine. In pre-licensure studies of the original Gardasil vaccine, Merck was permitted by the FDA to use Amorphous Aluminum Hydoxyphosphate Sulfate (AAHS), an aluminum adjuvant, in lieu of a saline placebo, as a control for nearly all of its trial participants. A reactive placebo, such as AAHS, has the ability to artificially increase the appearance of safety when used as a control in a clinical trial and neither the FDA nor Merck disclosed the amount of aluminum contained in the placebo. Both human and animal studies have shown that aluminum can enter the brain and that vaccine aluminum adjuvants can cause nerve cell death, as well as inflammation at the injection site leading to chronic muscle and joint pain and fatigue.
IMPORTANT NOTE: NVIC encourages you to become fully informed about HPV and the HPV vaccine by reading all sections in the Table of Contents , which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
What is the history of HPV vaccine use in America?

In the early 1980’s, studies confirmed the presence of HPV types 16 and 18 in cervical cancer cells, prompting research and development of a vaccine to prevent human papillomavirus (HPV). The FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) first discussed HPV vaccine and issues related to assessing effectiveness at its licensure at the November 2001 meeting.
Both Merck, the manufacturer of Gardasil and Gardasil 9, and GlaxoSmithKline, the manufacturer of Cervarix utilized new Virus-Like Particle (VLP) technology patented in 1994, to develop their HPV vaccines. VLPs contain particular proteins from the outside layer of the virus but lacks the genetic material to actually cause an infection. When injected, VLPs have the ability to produce an immune response due to the presence of foreign material.
Merck’s original Gardasil vaccine was the first FDA approved HPV vaccine. It targeted HPV types 6, 11, 16, and 18. Gardasil was granted Fast Track approval by the FDA after only a six month review process. According to the FDA, Fast Track approval is a program designed to accelerate the review of medications targeting “serious conditions and fill an unmet medical need.” To meet the criteria of “unmet need”, a drug must demonstrate a greater benefit over the currently available treatment.
Prior to Gardasil vaccination, prevention of cervical cancer included regular Pap smears and additional treatment options including colposcopy and removal of any abnormal lesions by techniques such as Laser Electrosurgical Excision Procedure (LEEP). These treatment options continue to be the standard of care for screening and prevention of cervical cancer and are credited with decreasing the U.S. cervical cancer by 75 percent.
Despite the availability of effective treatment options for the detection and prevention of cervical cancer, Gardasil was granted Fast Track status and an accelerated approval by the FDA. Accelerated approval is designed to allow drugs to be approved before they show any clinical benefit to the patient. Approval is based on findings associated with use of a “surrogate endpoint”, such as a physical marker, laboratory finding such as antibody levels or “other measure that is thought to predict clinical benefit, but is not itself a measure of clinical benefit.” In other words, Gardasil did not have to demonstrate true effectiveness – prevention of cervical cancer – prior to being determined to be effective and granted approval and licensed by the FDA.
Invasive cervical cancer from an unresolved HPV infection can take decades to develop, and as a result, Merck’s pre-licensing studies of Gardasil, limited to five years, could not clinically confirm that its vaccine would actually prevent cervical cancer. The FDA also permitted Merck to use Amorphous Aluminum Hydroxyphosphate Sulfate (AAHS), an aluminum adjuvant, in lieu of a saline placebo, as a control in pre-licensure clinical trials of the original Gardasil. The safety of aluminum adjuvants in vaccines had previously been called into question prior to use in HPV vaccines and continued research on aluminum hydroxide in vaccines found it to be associated with long-term cognitive dysfunction in addition to chronic pain and fatigue. Yet, even with studies that linked aluminum to inflammation and chronic health issues, Merck was granted permission to use it as a control in pre-licensure safety studies.
On May 18, 2006, Merck presented data to the FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC), which showed that Gardasil prevented cervical intraepithelial neoplasia (CIN) grades 1, 2, and 3 and, as a result, the FDA approved the vaccine for use in girls and women ages 9 through 26.
No clinical data to confirm Gardasil’s ability to prevent cervical cancer was available to VRBPAC. As a result, a “surrogate endpoint” – reduction in HPV related CIN 1, 2, and 3 related HPV types 16 and 18 – was used to infer whether the vaccine was likely to be effective. Using that metric, VRBPAC concluded that the vaccine was effective even though it had not been demonstrated to prevent cervical cancer. VRBPAC also voted to approve Gardasil for use in girls as young as 9 years of age even though very few girls between the ages of 9 and 15 were enrolled in pre-licensure studies.
Concerns about the state of science at the point of licensure and prior to being reviewed by the CDC were summarized in a June 2006 NVIC press release. Issues included clinical trial methods that involved use of bioreactive active placebos and known safety problems associated with injected aluminum, safety signals reported during pre-licensure clinical trials, inappropriate small sample sizes of the target population slated for vaccination, and an absence of proof of effectiveness.
Within weeks of Gardasil’s FDA approval, the CDC’s Advisory Committee on Immunization Practices (ACIP) voted to recommend three doses of the vaccine for all 11 and 12 year old girls with a “catch up” schedule for females between the age of 13 and 26. Though Gardasil was recommended for use prior to the onset of sexual activity, females who were currently sexually active were also encouraged to take it without prescreening for current HPV infection, even though pre-licensing data noted that the vaccine had the potential to exacerbate a current HPV infection. The CDC’s press release on Gardasil’s recommendation for routine use failed to report the vaccine’s inability to treat pre-existing HPV infections, pre-cancerous lesions or cervical cancer.
At the time of release, Gardasil was the most expensive vaccine in history, costing an average of $360 plus additional costs associated with medical office visits to complete the recommended three dose series. As a result, cost estimates to vaccinate all US females between the age of 11 and 18 years of age were pegged at $2 billion.
In 2005, one year prior to Gardasil’s FDA approval, over 1,500 Merck drug reps were redirected to focus on the Gardasil vaccine and Merck’s contributions to women’s health organizations and political campaigns increased substantially.
Following FDA licensure and ACIP recommendation, Merck launched a highly aggressive marketing campaign targeting teenage girls by encouraging them to be “one less” victim of cervical cancer. In addition to the advertising campaigns directed at young women, Merck began extensive state level lobbying campaigns to make HPV vaccination mandatory for school entry. Merck’s efforts included lobbying state legislators, drafting legislation, seeking support from female legislators and physician trade organizations, and pushing to be the primary source of information on HPV vaccination. By 2007, 41 states and the District of Columbia had introduced HPV vaccine legislation including bills in 24 states that would make HPV vaccination a requirement for entry into 6th grade.
In early 2007, HPV vaccine mandates and Merck’s legislative involvement was met with an enormous backlash from many consumer and family organizations that strongly opposed HPV vaccination mandates which were viewed as a violation of parental rights. Additionally, the Association of American Physicians and Surgeons and American Academy of Pediatrics expressed concerns related to safety, long-term effectiveness and reimbursement for such a costly vaccine.
Early February 2007, Texas Governor Rick Perry signed an executive order mandating the HPV vaccine for all 6th grade girls but this order was short-lived. Within three weeks of Perry’s decision to mandate the vaccine for school entry, the Texas House Committee on Public Health voted to rescind the executive order. The Senate followed suit and with both the Texas House and Senate’s overwhelming support of legislation to override the executive order, Perry opt to allow the override bill to become law. It was reported that Perry received $6,000 dollars in campaign contributions during his re-election campaign and one of the three Merck lobbyists in Texas previously worked as Perry’s chief of staff. Additionally, the mother-in-law of his chief of staff was also a state director for Women in Government, a national non-profit organization of female state legislators, that received substantial contributions from Merck to direct attention on cervical cancer, HPV infections, and Gardasil.
By late February 2007, Merck’s aggressive state legislative lobbying efforts had backfired and it publicly announced the end of its campaign aimed at mandating the HPV vaccine for girls entering the 6th grade. Merck cited public accusations that profits, not public health, were motivating its campaigns as its reason for ceasing efforts to make Gardasil a mandatory vaccine. Only the District of Columbia and the Commonwealth of Virginia enacted legislation requiring HPV vaccination for all 6th grade girls. These laws, however, provided parents with the option of declining the vaccine for their daughters. In 2015, Rhode Island became the third jurisdiction to enact HPV vaccination legislation by requiring vaccination for both girls and boys entering the 7th grade. Hawaii, in July 2020, became the fourth jurisdiction to require HPV vaccination for students entering 7th grade and higher. In July 2021, Virginia implemented an updated HPV vaccine policy to mandate that all boys and girls receive the vaccine as a condition of entry into 7th grade.
Reports of serious adverse events and deaths to the Vaccine Adverse Events Reporting System (VAERS) following vaccination with Gardasil began within weeks of FDA licensure and ACIP recommendation. In February 2007, NVIC released a press release and the first of three analyses of adverse reactions reported to VAERS. By May 2007, after being on the market for less than one year, VAERS had received 2,227 reports of serious adverse events following the administration of Gardasil. The early adverse reaction reports included seven deaths following receipt of Gardasil.
Additionally, an early NVIC analysis of VAERS data found a significantly greater risk of severe adverse events including Guillain-Barre Syndrome, respiratory and cardiac problems, central nervous system problems, convulsions, coordination and neuromuscular problems when Gardasil was administered along with another vaccine, Menectra, a meningococcal vaccine routinely administered to adolescents. No pre-licensure clinical trials evaluated safety when Gardasil was administered along with other vaccines targeted for use in teenagers.
In June 2008, Judicial Watch, a conservative, non-partisan foundation, promoting transparency, accountability and integrity in government, published a 25-page special report detailing Gardasil’s approval process, marketing practices, side-effects, and safety concerns. At the time their report was published, 8,864 adverse reactions following Gardasil vaccination had been reported to VAERS, including 18 deaths. Reported side effects included blood clots, Guillain-Barre Syndrome (GBS), growth of warts, dizziness, nausea, convulsions, and headaches. The report also highlighted reports of miscarriages noting that Gardasil was not studied in pregnant women and evaluated as to whether it could cause fetal harm.
Reports of serious reactions and deaths following HPV vaccination were also appearing in the media. These reports included seizures, paralysis, collapse, Guillain-Barre Syndrome as well as unexplained deaths. Yet, despite these concerns, the CDC continued to recommend the vaccine for all girls and young women, publicly denying any serious adverse events to be related to vaccination.
In July 2008, the CDC updated its Technical Instructions for the Medical Examination of Aliens in the United States and added the HPV vaccine as a requirement for all immigrant females between the age of 11 and 26, beginning August 1, 2008. While the update was intended to follow current guidelines requiring all immigrants to receive all appropriate CDC recommended vaccines, the update was criticized by many and on December 14, 2009, HPV vaccination was removed from the list of vaccines required for immigrants to the US.
To address the growing concerns raised by the public and medical professionals about Gardasil’s safety, in August 2009, the FDA and CDC posted a document on the FDA’s website that summarized the approval process and an overview of the safety monitoring process and findings to date.
Additionally, federal agency staff published the same information in a 2009 Journal of the American Medical Association (JAMA) article summarizing the Gardasil’s safety. After reviewing 12,424 VAERS reports the authors noted that 772 (6 percent of reports) were for serious events including 32 deaths. The article provided the reporting rates for syncope, local site reactions, dizziness, nausea, headache, hypersensitivity reactions, urticarial, venous thromboembolic events, autoimmune disorders and Guillain-Barre Syndrome, anaphylaxis, pancreatitis, transverse myelitis, motor neuron disease and death. The authors concluded that the rates of these events, except for syncope and venous thromboembolic events, were no different than background rates. The methods used and conclusions drawn for the analysis were criticized in a published letter to JAMA’s editor, which concluded that the reassurances of Gardasil’s safety were unsupported.
Merck continued to push for expanded use of its Gardasil vaccine and on October 16, 2009, the FDA approved Gardasil for use in boys and young men ages nine to twenty-six for the prevention of genital warts associated with HPV types 6 and 11, even though the vaccine had been studied in only about 3,000 males. Again, nearly all pre-licensing clinical trials failed to use an inert true placebo as a control and instead, used the novel bioactive aluminum adjuvant (Amorphous Aluminum Hydoxyphosphate Sulfate). While ACIP declined to recommend Gardasil for routine use in males, it did state that Gardasil could be administered to boys and men, ages 9 to 26 for the purpose of reducing the risk of developing genital warts associated with HPV types 6 and 11. Two years later, in October 2011, the ACIP voted to recommend routine vaccination with 3 doses of Gardasil for all boys ages 11-12 years with a catch up schedule for males ages 13 through 21 years.
Merck also submitted a request to the FDA for Gardasil to be approved in women between the ages of 27 and 45, but the FDA declined this request August 2010 due to a lack of data supporting substantial benefit for this population.
In October 2009 Cervarix, a bivalent recombinant vaccine manufactured by GlaxoSmithKline targeting HPV types 16 and 18, received FDA approval for use in girls ages 10 through 25 years of age for the prevention of CIN grades 1, 2 and higher, adenocarcinoma in situ, and cervical cancer. Cervarix contained a novel adjuvant named AS04 which is made of a lipid (MPL) and aluminum and had not previously been used in vaccines licensed in the U.S. In pre-clinical trials, the safety of Cervarix was assessed by comparing it to a licensed vaccine that is assumed to be safe - Hepatitis A which contained up to 500 mcg of Aluminum Hydroxide (AL(0H)3). Within days of FDA approval, the CDC’s Advisory Committee on Immunization Practices (ACIP) voted to recommend 3 doses of Cervarix for routine administration to girls ages 11 or 12, with a catch up schedule for females ages 13 through 26. However, by October of 2016, citing low demand for its product, GlaxoSmithKline announced that Cervarix would no longer be marketed in the United States.
In 2011, at the request of SaneVax, a non-profit organization promoting “only Safe, Affordable, Necessary & Effective vaccines and vaccination practices through education and information,” Sin Hang Lee, a Board Certified Pathologist, tested 13 different vials of Gardasil vaccine and discovered that each one contained Human Papillomavirus (HPV) DNA. In August 2011, SaneVax notified the FDA of these findings and requested an investigation and information about the effect of HPV DNA presence on vaccine safety. In response, on October 21, 2011, the FDA released a statement acknowledging the presence of HPV DNA fragments in the vaccine and stating that they regarded this as normal due to the vaccine’s manufacturing process rather than a vaccine contamination problem. No additional studies were recommended to determine whether the presence of HPV DNA in the vaccine posed any risks to vaccine recipients.
Severe adverse reactions following HPV vaccination continued to be reported in the United States and abroad. In June of 2013, the Japanese government withdrew their support of HPV vaccination, citing safety concerns related to the high number of serious adverse reactions reported following vaccination. Three years later, in July of 2016, Japanese victims of HPV vaccination launched a class action lawsuit against the Japanese government, Merck, the maker of Gardasil, and GlaxoSmithKline, the maker of Cervarix, for damages related to the numerous health problems suffered post-vaccination. Japan relaunched its HPV vaccine campaign in spring of 2022, after a national survey reportedly concluded that unvaccinated girls suffered symptoms that were attributed to HPV vaccine at a similar rate as those who received the vaccine.
American journalist Katie Couric, profiled HPV vaccination during a December 2013 segment of her TV talk show, Katie. The program discussed the benefits and risks of vaccination and interviewed two mothers who reported sudden serious health issues that followed their daughters HPV vaccinations. Couric’s program resulted in an onslaught of attacks from numerous media sources accusing her of promoting junk science and fear mongering. After enduring several days of negative media stories, Couric published a blog commentary in the Huffington Post stating that the show should have focused more on the vaccine’s safety and efficacy, and less on the “serious adverse events that have been reported in very rare cases following the vaccine.”
While mainstream media in the US has effectively silenced nearly all discussion questioning the safety of HPV vaccination, reports in other countries have continued to surface.
In March 2015, one of Denmark’s national television stations aired a documentary focusing on the serious side effects reported following HPV vaccination and profiling the stories of three young women who developed chronic health problems following HPV vaccination. At the request of Danish scientists and clinicians, the European Medicines Agency (EMA), the agency responsible for safety monitoring and scientific evaluation of drugs and vaccines in Europe, reviewed two commonly reported side effects of HPV vaccination, postural orthostatic tachycardia syndrome (POTS) and complex regional pain syndrome (CRPS), and determined that no link existed between these symptoms and HPV vaccination. However, several scientists and doctors objected to the report, expressing concerns over conflicts of interest and use of inappropriate methods that relied on previously published data.
Ireland’s TV3 television station aired a similar documentary December 2015 profiling several girls who developed debilitating health problems following HPV vaccination. In 2017, Sacrificial Virgins, a United Kingdom documentary which discussed the lack of evidence proving that HPV vaccination prevented cervical cancer, the reports of debilitating side effects and death, and the HPV vaccine litigation in Japan, Spain, and Columbia was shown at several independent film festivals. Despite numerous reports of serious side effects and death following HPV vaccination globally, in most cases, government health agencies have continued to actively promote HPV vaccine use and contend that the vaccine is effective and is not linked to any serious side effects or death.
Over time, evidence that Merck’s original Gardasil vaccine formulation was inadequate in addressing all the concerning HPV types became apparent. In response, Merck increased the number of types of HPV in its vaccine from four to nine, and on December 10, 2014, its 9-valent recombinant vaccine (Gardasil 9) received FDA approval for use in females ages 9 through 26 years of age, for the prevention of genital warts associated with HPV Types 6 and 11 and for the prevention of anal, cervical, vaginal, and vulvar cancers associated with HPV Types 16, 18, 31, 33, 45, 52, and 58. The FDA also approved Gardasil 9 for use in males ages 9 through 15 for the prevention of genital warts associated with HPV Types 6 and 11 and for the prevention of anal cancer associated with HPV Types 16, 18, 31, 33, 45, 52, and 58. In addition to doubling the amount of HPV protein antigen contained in the original Gardasil, Merck’s Gardasil 9 increased the amount of bioactive aluminum adjuvant to from 225 mcg to 500mcg.
As previously noted for Gardasil, pre-licensure studies of Gardasil 9 did not use true placebos as controls and instead, tended to compare Gardasil 9 to Gardasil. In February 2015, the CDC’s ACIP voted to recommend three doses of Gardasil 9 to be administered to all males and females ages 11-12, with a catch up dosing for females between the age of 13 and 26, males between the age of 13 and 21, and select high-risk males up to the age of 26 years of age. Gardasil 9 was recommended by the ACIP in males between the age of 13 and 21 even though the FDA had yet to approval its use in males over 15 years of age. One year later, the FDA approved Gardasil 9 for use in males ages 16 to 26 in December 2015.
In December 2016, two years after Gardasil 9 was licensed by the FDA, ACIP voted to decrease the recommended 3-dose schedule to a 2-dose schedule for HPV vaccination in females and males between the ages of 9 and 14 where the second dose was to be administered six to twelve months following the initial dose. The 3-dose vaccine schedule continued to be recommended for individuals receiving the first HPV dose after the age 15. The ACIP stated that the dosing changes were based on evidence that the immune response in persons between the age of 9 and 14 after two doses of HPV vaccine was significant enough to produce adequate long-lasting antibodies against HPV types present within the vaccine. At the same time, Gardasil 9 became the only available HPV vaccine on the market in the US. The CDC recommended using “any HPV vaccine at the recommended dosing schedule” to complete the vaccination schedule for vaccines that were no longer available even though no studies existed to support the use of “a mixed regimen of HPV vaccines”.
Despite Merck’s 2016 marketing campaign, which appeared to shame parents for declining Gardasil for their children, HPV vaccination uptake rates remained low. A 2018 published report on HPV vaccination rates in adolescents completed by the insurance company Blue Cross Blue Shield found that in 2016, only 34 percent of teenagers had received their first dose of HPV vaccine by the age of 13. This study also found that only 9 percent of adolescents had completed the series prior to the age of 13. An additional survey of over 700 parents whose children had not received the HPV vaccine reported that over half of the parents were not planning to have their child receive HPV vaccination and 60 percent of these parents cited vaccine safety concerns as the reason they decided to decline HPV vaccination for their child.
In June 2018, the FDA granted Merck a priority review of its application to expand Gardasil 9 use in both males and females 27 to 45 years of age. Previously, the FDA reviewed data for women ages 27 to 45, in August 2010 and concluded that the vaccine showed no significant benefit to this population.
In October 2018, the FDA approved Gardasil 9 for use in both males and females up to age 45, and in June 2019, ACIP voted to recommend use of the vaccine in males and females 27 through 45 through ‘shared clinical decision-making’ – a process that physicians can use to determine who might benefit from vaccination within this population.
For females over 21 years of age, HPV vaccination does not replace the need for routine Pap smear testing to detect abnormal cells that can cause cervical cancer especially considering that cervical cancer can be caused by HPV types not included in the vaccine. The effectiveness of HPV vaccination is still unknown and according to the CDC, “it may take decades to see population-level impact” from this vaccine.
IMPORTANT NOTE: NVIC encourages you to become fully informed about HPV and the HPV vaccine by reading all sections in the Table of Contents , which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
How effective is HPV vaccine?

As of April 2023, there are no studies that confirm HPV vaccine has reduced the incidence of HPV associated cancers. According to CDC data, HPV associated cancers increased to 41,000 cases between 2010 to 2014 from 26,000 cases between 2004 and 2008. From 2011 to 2015, HPV associate cancers increased again to 42,700 cases, with approximately 24,400 among women and 18,300 among men. During the CDC’s Advisory Committee on Immunization Practices (ACIP) February 2018 meeting, it was reported that “it may take decades to see population-level impact due to the length of time between the initial HPV infection and the development of cancer.”
HPV vaccines cannot treat existing HPV infections and does not protect against HPV types not covered by the vaccine. In fact, 21 percent of HPV associated cancers involve HPV types not included in Gardasil 9. HPV vaccine does not replace the need for routinely recommended cervical or anal cancer screenings. If vaccinated women opt to skip routine cervical cancer screening, cervical cancer rates are expected to increase. Cervical cancer has been reported in women who have received HPV vaccine.
Long-term effectiveness studies of Gardasil found the vaccine to be between 88.4 and 94.4 percent effective against HPV Type 6; 89.1 and 95.5 percent effective against HPV Type 11; 96.8 to 99.1 percent effective against HPV Type 16; and 60 to 64.1 percent effective against HPV Type 18 after nine years. A 2017 Merck funded study of five year Gardasil 9 antibody levels and concluded that the vaccine was 97.4 percent effective against the HPV Types covered by the vaccine. Research studies indicate that, unless HPV vaccination produces high antibody titers for at least 15 years, vaccination alone will not prevent cervical cancer.
A 2015 study found that HPV vaccinated women had a higher rate of high-risk HPV types other than HPV Type 16 and 18, which put them at an increased risk of cervical and other HPV related cancer. Additionally, a study conducted in Costa Rica also found that women who received HPV vaccine were more likely than unvaccinated women to develop HPV from strains not covered in the vaccine.
A 2020 published study that reviewed 12 HPV vaccine clinical trials on Gardasil and Cervarix vaccines found that the trial design may have led to an overstatement of vaccine efficacy. Additionally, researchers also found that the clinical trials did not adequately represent populations that were targeted for the vaccine. While clinical trials showed evidence that the vaccines could prevent CIN1 lesions, this finding was insignificant because these lesions generally resolve without the need for intervention.
In the US, researchers have not found any association between vaccination and cancer rates. Decreases in cervical cancer rates were noted even prior to the introduction of Gardasil in 2006, and the rates have essentially remained the same through 2020. A study published in 2022 in the International Journal of Gynecological Cancer found that:
“Over the last 18 years [2001 through 2018, 29,715 women were diagnosed with distant stage cervical carcinoma … When examining the trends over time, there has been an annual increase in distant stage cervical cancer at a rate of 1.3% per year. The largest increase is seen in cervical adenocarcinoma with an average annual percent change of 2.9%.”
In Australia, there has been a 30 percent increase in cervical cancer rates among 30 to 34-year-old women in the past 13 years despite the introduction and widespread use of the Gardasil vaccine. Similarly, in the United Kingdom, which introduced HPV vaccination in 2008, rates of cervical cancer among 20 to 24-year-old women had increased 70 percent by 2016.
While cervical cancer associated with HPV types found in the vaccine have reportedly decreased, the rate of HPV-related cancers from any type of HPV strain have increased significantly in recent years. In 2015, 43,000 individuals developed an HPV-related cancer, up from 30,000 in 1999.
IMPORTANT NOTE: NVIC encourages you to become fully informed about HPV and the HPV vaccine by reading all sections in the Table of Contents , which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
Can HPV vaccine cause injury & death?

The most commonly reported side effects of HPV vaccination include pain, swelling, and redness at the injection site, nausea, headache, fever, fatigue, and muscle or joint pain. Fainting – referred to as a syncopal episode - following HPV vaccination has been frequently reported and as a result, it is recommended that individuals receiving the vaccine remain sitting or lying down to prevent syncope and any potential injuries that may result from a fall. However, more severe reactions have also been reported in HPV vaccine clinical trials and to the federal Vaccine Adverse Events Reporting System (VAERS).
Some of the adverse events reported by the manufacturers during pre-licensing clinical trials included:
Gardasil - injection site pain, swelling, redness and bruising, fever, headache, nausea, dizziness, syncope, sometimes in conjunction with seizure-like activity, anaphylaxis, diarrhea, vomiting, cough, upper respiratory tract infection, nasal congestion, insomnia, malaise, oropharyngeal pain, nasopharyngitis, upper abdominal pain, gastroenteritis, appendicitis, pelvic inflammatory disease, urinary tract infection, pneumonia, pulmonary embolism, pyelonephritis, bronchospasm, and death.
Cervarix - injection site pain, redness, bruising and swelling, syncope, fatigue, headache, gastrointestinal symptoms, rash, fever, arthralgia, myalgia, urticarial, urinary tract infection, back pain, dysmenorrhea, nasopharyngitis, influenza, vaginal infection, pharyngitis, chlamydia infection, arthritis, rheumatoid arthritis, Celiac disease, diabetes mellitus, erythema nodosum, inflammatory bowel disease, hyperthyroidism, hypothyroidism, multiple sclerosis, transverse myelitis, systemic lupus erythematosus, thrombocytopenia, vasculitis, optic neuritis, vitiligo, and death.
Gardasil 9 - injection site pain, swelling, redness, and bruising, syncope, fever, headache, nausea, dizziness, fatigue, diarrhea, upper respiratory tract infection, upper abdominal pain, oropharyngeal pain, myalgia, asthmatic crisis, anaphylaxis, and death.
Gardasil 9 reported post marketing adverse events include: pulmonary embolus, idiopathic thrombocytopenic purpura, lymphadenopathy, autoimmune hemolytic anemia, pancreatitis, asthenia, chills, fatigue, malaise, bronchospasm, urticarial, anaphylaxis, acute disseminated encephalomyelitis, dizziness, transverse myelitis, Guillain-Barré syndrome, headache, motor neuron disease, paralysis, seizures, syncope (including syncope associated with other seizure-like activity and tonic-clonic movements) sometimes resulting in injury from falling, deep vein thrombosis, cellulitis, myalgia, arthralgia, and death.
In 2007, NVIC reviewed adverse events reported to VAERS and noted a statistically significant increased risk of Guillain-Barre Syndrome(GBS) and other serious adverse events when Gardasil was administered with other vaccines, especially the meningococcal vaccine, Menactra. The analysis noted a 1,130 percent increase in GBS, a 674 percent increase in injuries from falls after loss of consciousness, a 234 percent increase in coordination and neuromuscular problems, a 118 percent increase in cardiac problems, a 114 percent increase in respiratory problems and a 30.1 percent increase in convulsions and central nervous system problems when Gardasil was administered with Menactra.
During the past decade, there have been numerous studies and reports linking HPV vaccination to chronic illnesses in children and young adults. These include anaphylaxis, lupus, erythema multiforme, acute disseminated encephalomyelitis, transverse myelitis, amyotrophic lateral sclerosis (ALS), central nervous system demyelination, multiple sclerosis, including pediatric multiple sclerosis, Guillain-Barre Syndrome, pancreatitis, inflammatory bowel syndrome, brachial plexus neuritis, brachial neuritis, optic neuritis, neuromyelitis optica, opsoclonus myoclonus, evanescent white dot syndrome, acute cerebellar ataxia, autoimmune hepatitis, autoimmune neuromyotonia, vasculitis, thrombocytopenic purpura, immune thrombocytopenic purpura, Postural Orthostatic Tachycardia Syndrome (POTS), Complex Regional Pain Syndrome (CRPS), Chronic Fatigue Syndrome (CFS), and peripheral sympathetic nerve dysfunction.
A published questionnaire of HPV vaccination recipients focusing on a combination of chronic illness including POTS, CRPS, and fibromyalgia found that 93 percent of individuals reporting symptoms related to these conditions were still incapacitated and unable to work or attend school four years after vaccination. Additionally, several studies have linked HPV vaccination to primary ovarian failure resulting in impaired fertility . A 2018 paper published in the Journal of Toxicology and Environmental Health found lower pregnancy rates in women who had received HPV vaccination. This paper, however, was retracted by the journal based on “several public and private expressions of concern about flaws in analysis.” No detailed explanation of the reason for the retraction was ever provided to the paper’s author.
Adverse events following HPV vaccination have also been linked to a relatively new medical condition termed Autoimmune/inflammatory Syndrome Induced by Adjuvants (ASIA). In 2011, Dr. Yehuda Shoenfeld, the founder and head of the Zabludowicz Center for Autoimmune Diseases in Israel, published a paper associating four medical conditions - Gulf War syndrome (GWS), macrophagic myofasciitis syndrome (MMF) (a syndrome previously related to the use of aluminum adjuvants), siliconosis (a condition related to silicone breast implants) and post-vaccination phenomena (chronic illness following vaccination) to a previous adjuvant exposure.
Dr. Shoenfeld noted that patients suffering from these conditions presented with very similar clinical symptoms. Since then, published studies have linked the aluminum adjuvant found in the HPV vaccine to several chronic health conditions including postural tachycardia syndrome (POTS), primary ovarian failure (POF), chronic epipharyngitis, pseudo-neurological syndrome, and severe somatoform and dysautonomic syndromes. An epidemiological study of data collected from VAERS estimated that 3.6 out of 100,000 doses of HPV vaccination resulted in symptoms that were consistent with a diagnosis of ASIA.
An animal study on the effects of HPV vaccination found that both the HPV antigens and the aluminum adjuvant appear to have the ability of trigger autoimmune reactions and neuroinflammation in female mice, leading to changes in behavior patterns.
A phenomenon known as immune cross-reactivity is also believed to play a role in health issues following HPV vaccination. With this condition, the immune system is unable to differentiate between human proteins and the HPV antigens because of molecular similarities, known as molecular mimicry. This cross-reactivity is thought to have the capability of triggering a number of auto-immune diseases. In this paper, researchers studied epitopes (part of the antigen that is responsible for stimulating the immune response) of 15 different types of HPV, including eight of the nine types found in the Gardasil 9 vaccine and compared them to human proteins. The researchers confirmed that there was an extremely high probability of peptide sharing between human proteins and HPV epitopes and should cross-reactivity occur, a number of significant auto-immune disorders could occur, and may include cardiac and circulatory issues, neuropsychiatric disorders, lupus, reproductive disorders including ovarian failure, and sudden death.
Studies linking HPV vaccination to sudden death in previously healthy women have also been published. A 2012 published case study of two deaths following HPV vaccination concluded that the HPV-16L1 antigens present in HPV vaccines have the potential to cause fatal autoimmune vasculopathies. Also in 2012, Sin Hang Lee, a research scientist and board-certified pathologist, published a case study involving the sudden and unexplained death of a young women six months after completing a three-dose series of Gardasil. Dr. Lee found in the blood and spleen HPV-16 gene DNA that were similar to HPV-16 gene DNA fragments in Gardasil. The HPV-16 LI gene DNA was bound to the same aluminum adjuvant found in the vaccine, which protected it from degradation. It continues to be unknown whether or not these HPV DNA fragments played a role in the girl’s death.
A 2017 article published in Drug Safety reviewed safety concerns associated with HPV vaccination. Data reported to adverse reaction reporting systems from several countries were analyzed and found to contain relatively high numbers of reports for headache, dizziness, fatigue, and syncope associated with prolonged hospitalization or debilitation. While some of the reports listed Postural Orthostatic Tachycardia Syndrome (POTS), Complex Regional Pain Syndrome (CRPS) or Chronic Fatigue Syndrome (CFS) as a diagnosis, the vast majority of the reports lacked any diagnosis. This study also found significantly higher number of events involving a combination of dizziness and headache with either syncope or fatigue following HPV vaccination compared to adverse reactions of other vaccines. It was also noted that these combinations of symptoms were first reported in countries that were the earliest to approve and recommend HPV vaccination and that reported symptoms persisted globally.
As a result of these findings, questions have persisted to whether current drug and vaccine safety monitoring tools have the capability to adequately detect and respond to signals indicating that a serious problem may exist with a product currently on the market. A 2018 study found that only about half of the available clinical trials involving HPV vaccines had been completed before the vaccines were approved by both the Federal Drug Administration (FDA) and the European Medicines Agency (EMA). This study also noted that drug manufacturers only published the results of about two-thirds of the HPV clinical trials, leaving the study’s authors to question whether drug manufacturers were selectively choosing which clinical data to publish.
In December 2017, Slate Magazine published a cover story on the pre-licensure clinical trials of the Gardasil vaccine. This investigational report determined that Merck’s pre-licensure safety studies “used a convoluted method that made objective evaluation and reporting of potential side effects impossible during all but a few weeks of its years long trial.” The article noted that Merck’s clinical trial investigators were permitted to use personal judgment when reporting medical problems as an adverse event, essentially allowing study investigators to decide what symptoms might possibly be related to vaccination. Study investigators were also allowed to list new health issues following vaccination as medical history, not adverse events, and limited safety follow up to 14 days following each of the three doses of Gardasil vaccination. Slate’s investigation located several women involved in Gardasil’s pre-licensure trials who reported chronic illness post-vaccination to study investigators, yet their symptoms were never reported by Merck.
In April 2018, the Indian Journal of Medical Ethics published a report suggesting that Sweden’s increase in cervical cancer rates might be associated with HPV vaccination. The study’s author, concerned that he may be targeted for questioning a vaccine’s safety or efficacy, chose to publish under an assumed name without contacting the journal in advance. Initially, the journal chose to allow the article to be published despite the deception after determining that the author had both the necessary credentials and faced a credible threat of harm, stating “the issues raised by it are important and discussion on it is in the public interest.” However, two weeks later, after receiving “valuable advice from the journal’s editorial board and others”, the article was retracted. The journal, however, stated that they “hope that the hypothesis of possible harm of vaccinating women previously exposed to HPV is carefully explored in future studies.” Data from Gardasil’s pre-licensure clinical trials had previously demonstrated a higher incidence of cervical intraepithelial neoplasia (CIN) grade 2 and 3 in women previously infected with the particular strain targeted by the vaccine.
Using the MedAlerts search engine, as of June 30, 2023, the federal Vaccine Adverse Events Reporting System (VAERS) contains more than 74,229 reports of HPV vaccine reactions, hospitalizations, injuries and deaths and includes 628 related deaths, 7,484 hospitalizations, and 3,578 disabling conditions. Over 55 percent of the reported serious adverse events occurred in children and teens under 17 years of age. However, the number of HPV vaccine related injuries and deaths reported to VAERS is assumed to underreported as explained below.
Even though the National Childhood Vaccine Injury Act of 1986 legally required pediatricians and other vaccine providers to report serious health problems following vaccination to the federal vaccine adverse event reporting system (VAERS), many doctors and other health care providers giving vaccines to children and adults fail to report vaccine-related health problem to VAERS. The evidence suggests that only one to 10 percent of serious health problems that occur after use of prescription drugs or vaccines in the U.S. are ever reported to federal health officials who are responsible for regulating the safety of drugs and vaccines and issue national vaccine policy recommendations.
As of August 1, 2023, 822 claims have been filed to the federal Vaccine Injury Compensation Program (VICP) for 22 deaths and 800 injuries that occurred after HPV vaccination. To date, the U.S. Court of Claims has compensated 169 children and adults who filed claims for HPV vaccine injuries.
For example, an HPV vaccine injury claim was filed and awarded by the VICP for Christina Tarsell. Christina was a 21-year-old college student majoring in studio arts at Bard College when she received a series of three Gardasil shots. A talented athlete, artist and honor roll student, she died suddenly and without explanation shortly after the third shot in June 2008. Ten years later, in 2018, the government conceded the case and awarded compensation to her mother for Christina’s vaccine-related death.
IMPORTANT NOTE: NVIC encourages you to become fully informed about HPV and the HPV vaccine by reading all sections in the Table of Contents , which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
Who is at highest risk for complications from HPV vaccine?

The Institute of Medicine has acknowledged that there is individual susceptibility to vaccine reactions for genetic, biological and environmental reasons but that vaccine providers cannot accurately predict prior to a vaccine’s administration who will suffer complications, injury or death from vaccination. However, a person who has previously had a serious reaction to a vaccination or is acutely or chronically ill should become informed about all potential risks associated with vaccination and discuss any concerns with a trusted health care professional before receiving HPV vaccine or any other vaccine.
A 2013 study examining an association between systemic lupus erythematosus (SLE) or SLE-like disease and HPV vaccination noted that individuals with a personal or family history of auto-immunity or those who had previously reacted to HPV vaccination had a higher risk of developing an auto-immune disorder post vaccination. A 2017 case report published in Clinical Pediatrics reported a case of neurocardiogenic syncope and postural orthostatic tachycardia syndrome in a previously healthy 11-year-old female with a family history of autoimmune disease post Gardasil vaccination.
Currently, a history of a severe allergy to a vaccine component, including yeast, history of a life-threatening allergic reaction to a previous HPV shot, and pregnancy are the only CDC approved official contraindications (medical reasons for not getting vaccinated) to receiving HPV vaccines. However, the CDC also states that HPV vaccination should be postponed if a person is “moderately or severely ill.”
IMPORTANT NOTE: NVIC encourages you to become fully informed about HPV and the HPV vaccine by reading all sections in the Table of Contents , which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
Who should not get HPV vaccine?

According to the CDC, anyone who has ever had a life-threatening allergic reaction to a previous dose of HPV vaccine should not get another dose. Anyone with a severe allergy to any part of the vaccine should not receive the vaccine. The CDC also advises that anyone with any severe allergies, including a severe allergy to yeast, should notify their doctor prior to vaccination. HPV vaccine is not recommended for pregnant women. People who are moderately or severely ill at the time the vaccine is scheduled should wait until they recover before getting HPV vaccine.
While the CDC states that HPV vaccine may be administered to women who are breastfeeding, the manufacturer’s product insert reports that there are insufficient studies available to assess the effects of the vaccine on milk production/excretion or on the breastfed infant. Gardasil 9, the only HPV vaccine currently available in the United States, is approved for males and females between the ages of 9 and 45. Persons younger than 9 or older than 45 should not receive this vaccine.
Information about contraindications (reasons why a person should not get a vaccine) to HPV vaccine are contained in the manufacturer’s product information package insert that accompanies vials of vaccine provided to doctors and other medical personnel administering the vaccine, and can also be found on the FDA’s website.
The Institute of Medicine has acknowledged that there is individual susceptibility to vaccine reactions for genetic, biological and environmental reasons but that vaccine providers cannot accurately predict prior to a vaccine’s administration who will suffer complications, injury or death from vaccination.
IMPORTANT NOTE: NVIC encourages you to become fully informed about HPV and the HPV vaccine by reading all sections in the Table of Contents , which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
What questions should I ask my doctor about the HPV vaccine?

NVIC’s If You Vaccinate, Ask 8! webpage and downloadable brochure suggests asking eight questions before you make a vaccination decision for yourself, or for your child. If you review these questions before your appointment, you will be better prepared to ask your doctor questions. Also make sure that the nurse or doctor gives you the relevant Vaccine Information Statement (VIS) for the vaccine or vaccines you are considering well ahead of time to allow you to review it before you or your child gets vaccinated. Copies of VIS for each vaccine are also available on the CDC's website and there is a link to the VIS for HPV vaccine on NVIC's “Quick Facts” page.
Due to the brevity of the VIS, it is also a good idea to read the vaccine manufacturer product insert that can be obtained from NVIC’s HPV Quick Facts, doctor or public health clinic to get additional information. Federal law requires drug companies marketing vaccines to include certain kinds of vaccine benefit, risk and use information in product information inserts that may not be available in other published information, like the VIS.
Other questions that may be useful to discuss with your doctor before getting the HPV vaccine are:
- If other vaccines in addition to HPV vaccine are scheduled for my child at this office visit, am I allowed to modify the schedule so fewer vaccines are given at once?
- What are the possible side effects that may occur following vaccination?
- What should I do if my child has a high fever or appears very ill after vaccination?
- What other kinds of reaction symptoms should I call to report after HPV vaccination?
- Are there other options for preventing HPV infection?
It also is important to be able to recognize a vaccine reaction and seek immediate medical attention if the reaction appears serious, as well as know how to make a vaccine reaction report to federal health officials at the Vaccine Adverse Reporting System (VAERS). NVIC’s Report Vaccine Reactions—It’s the Law webpage can help you file a vaccine reaction report yourself to VAERS if your doctor fails or refuses to make a report.
IMPORTANT NOTE: NVIC encourages you to become fully informed about HPV and the HPV vaccine by reading all sections in the Table of Contents , which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
NVIC Press Releases, Statements, and Reports
NVIC Statements & Commentaries
Commentaries & Statements
- Gardasil Vaccine, Katie Couric & Cyber-Lynching. Fisher, B.L. Jan. 29, 2014.
- Gardasil & Swine Flu Vaccine: Inconvenient Truths. Fisher, B.L. Aug. 24, 2009.
- Preventing Gardasil Injuries & Deaths. Fisher, B.L. Jul. 14, 2009.
- Gardasil Death & Brain Damage: A National Tragedy. Fisher, B.L. Feb. 9, 2009.
- Letter: NVIC HPV Vaccine Safety Analysis Part III to Advisory Committee on Immunization Practices (ACIP) of Centers for Disease Control. Aug. 14, 2007.
Special Reports
- Analysis of Gardasil & Menactra Adverse Event Reports to the Vaccine Adverse Events Reporting System (VAERS) - Feb. 2009
- Analysis of Gardasil Vaccine Adverse Events Part III. Fisher, B.L., Debold, V., Downey, C. - Aug. 15, 2007
- NVIC Report/Analysis of Gardasil Vaccine Adverse Events Part II. Debold, V., Fisher, B.L. Feb. 21, 2007.
- NVIC Analysis of Gardasil Vaccine Adverse Events Part I. Debold, V., Fisher, B.L. Feb. 1, 2007.
- Survey of GARDASIL Costs in Private Pediatricians Offices. Feb. 1, 2007.
Press Releases
- NVIC Analysis Shows Greater Risk of GBS Reports When HPV Vaccine Is Given with Meningococcal and Other Vaccines. Aug. 15, 2007.
- Vaccine Safety Group Releases GARDASIL Reaction Report. Feb. 21, 2007.
- HPV Vaccine Mandates Risky and Expensive. Feb. 1, 2007.
- Merck's GARDASIL Not Proven Safe for Little Girls NVIC. Jun. 27, 2006.
The Vaccine Reaction
- Baker A. More U.S. Parents Reject HPV Vaccine Over Safety Concerns. June 5, 2023.
- TVR Staff. Young Girl Dies in Thailand Following HPV Vaccination. The Vaccine Reaction Sept. 14, 2020.
- TVR Staff. American Cancer Society Tells Doctors to Give Nine-Year-Old Children HPV Vaccine The Vaccine Reaction July 19, 2020.
- TVR Staff HPV Vaccine Mandated for All 10-Year-Old Girls in Costa Rica The Vaccine Reaction June 19, 2019.
- La Vigne P Blockbuster Vaccine Sales Drive Higher Earnings for Merck The Vaccine Reaction May 22, 2019.
- La Vigne P Blacklisting of Gardasil Studies to Silence the Safety Debate? The Vaccine Reaction Mar. 20, 2016.
- La Vigne P Gardasil 9 Vaccine Prompts Push for National Program The Vaccine Reaction June 22, 2016.
- Raines K FDA Expands the Market for Gardasil Vaccine Use The Vaccine Reaction Nov. 22, 2018.
- La Vigne P Investigation Questions Gardasil Clinical Trial Safety Data The Vaccine Reaction Jan. 10, 2018.
- TVR Staff Irish Girls Harmed by Gardasil Vaccine The Vaccine Reaction Oct. 12, 2017.
- Dworskin C Are Aluminum Adjuvants Plus Gardasil a Uniquely Damaging Neuroinflammatory Cocktail? The Vaccine Reaction Aug. 5, 2016.
- TVR Staff American College of Pediatricians Warns About Gardasil Risk The Vaccine Reaction Feb. 4, 2016.
- Walia A Girl’s Ovaries Destroyed by Gardasil: Merck Did Not Research Effects of Vaccine on Female Reproduction The Vaccine Reaction Apr.12, 2017.
- TVR Staff Study Linking Gardasil to Behavioral Abnormalities Pulled from Vaccine Journal The Vaccine Reaction Mar. 12, 2016.
- Austin V Gardasil Devastation is NO Coincidence The Vaccine Reaction July 20, 2016.
- TVR Staff Gardasil-Injured Teen in Ireland Gets No Help from Country’s Public Health Service The Vaccine Reaction June 6, 2016.
- La Vigne P HPV Vaccines Raise International Red Flags The Vaccine Reaction Oct. 7, 2015.
- Tarsell E Gardasil Was Linked to My Daughter’s Death The Vaccine Reaction Sept. 19, 2018.
- La Vigne P European Agency Declares HPV Vaccines Safe, But Denmark, Japan Skeptical The Vaccine Reaction Dec. 4, 2015.
- Holland M, Mack Rosenburg K, Iorio E The HPV Vaccine on Trial: Seeking Justice for a Generation Betrayed The Vaccine Reaction Oct. 18, 2018.
- Jackson V Irish Philanthropist Jonathan Irwin Vows to Stand Up for Gardasil-Injured Girls The Vaccine Reaction July 11, 2017
- Raines K, Fisher BL Mass HPV Vaccination Plan for the U.S. The Vaccine Reaction Nov. 14, 2018
- Parpia R Victims of HPV Vaccine in Japan Will Sue State and Vaccine Makers The Vaccine Reaction Apr. 13, 2016.
- Rosenberg M Vaccines Safe Say Those Who Gave Us Vioxx, Bextra, Baycol, Trovan, Phen-Fen, Xarelto, Raxar, Seldane… The Vaccine Reaction Feb. 1, 2017.
- Heimer M The HPV Vaccine: What You Need to Know About the Dumbest Vaccine Ever The Vaccine Reaction Mar. 5, 2016.
- TVR Staff Illinois Teen Dies of ADEM Three Weeks After HPV Vaccination The Vaccine Reaction Sept. 12, 2018.
- TVR Staff Girls in South Africa Seriously Ill After Getting HPV Vaccine The Vaccine Reaction May 14, 2017.
- Parpia R How Doctors Talk to Patients Impacts HPV Vaccination Rates The Vaccine Reaction Nov. 19, 2015.
- Parpia R Japan, U.S. Poles Apart on Harmful HPV Vaccine The Vaccine Reaction June 9, 2015.
- The Vaccine Reaction Staff Drug Companies Pay FDA and NIH to Fast Track and Market Vaccines The Vaccine Reaction Sept. 28, 2018.
- Newman A Uprising Against HPV Vaccine Mandate Sweeps Rhode Island The Vaccine Reaction Aug. 27, 2015.
- Raines K HPV Study Finds Higher Risk of Cervical Cancer Deaths in Older Black Women The Vaccine Reaction Feb. 8, 2017.
- Attkisson S What You Didn’t Know About a Doctor’s Stance on the HPV Vaccine The Vaccine Reaction July 1, 2015.
- McGovern C Vaccine Boom, Population Bust The Vaccine Reaction Oct. 4, 2018.
- La Vigne P Nordic Cochrane Center Files Complaint About Scientific Misconduct, Secrecy in HPV Vaccine Probe in Europe The Vaccine Reaction Aug. 10, 2016.
- TVR Staff HPV Vaccine Mandate Rejected by Allegheny County Board of Health The Vaccine Reaction July 15, 2016.
- Dworksin C Flawed HPV Vaccine Safety Statement Triggers Butterfly Effect The Vaccine Reaction Feb. 4, 2016.
Videos
- Ireland Fights HPV Vaccine Injury & Pharma Control The Vaccine Reaction May 16, 2016.
- Sisters Sue Over HPV Vaccine—The Doctors The Vaccine Reaction Dec. 28, 2016.
- Gardasil: One Mom’s Story The Vaccine Reaction Sept. 18, 2018.
- Guard Yourself: A Gardasil Documentary Short The Vaccine Reaction Jan. 4, 2017.
- Is There Objective Evidence HPV Vaccination Programs are Unjustified? The Vaccine Reaction Oct. 6, 2016.
IMPORTANT NOTE: NVIC encourages you to become fully informed about HPV and the HPV vaccine by reading all sections in the Table of Contents , which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
Analysis of Vaccine Adverse Events Reporting System Reports Part I
Adverse Reactions, Concerns and Implications
Human Papilloma Virus Vaccine Safety
Analysis of Vaccine Adverse Events Reporting System Reports Part I
Adverse Reactions, Concerns and Implications
by Vicky Debold, PhD, RN and Barbara Loe Fisher
Feb. 1, 2007
On June 8th 2006, the Food and Drug Administration (FDA) announced the approval of GARDASIL, and on June 29th the Advisory Committee on Immunizations Practices (ACIP) voted to recommend adding GARDASIL human papilloma virus vaccine to the Centers for Disease Control's national childhood recommended immunization schedule. On July 14th the first report of a serious reaction to the vaccine was filed with the federal Vaccine Adverse Event Reporting System (VAERS).
A 16-year-old Illinois girl was vaccinated July 7th and 13 days later developed symptoms eventually diagnosed as Guillian-Barre Syndrome. A 14-year-old girl in the District of Columbia was vaccinated on July 11th and complained of severe pain immediately following the injection, fell off the examining table and experienced a 10 to 15 second fainting spell ending up in the emergency room with a headache and speech problems. The report of this reaction, the first in the nation, was filed on July 14th, 15 days after the ACIP vote.
Six months later, 82 reports of GARDASIL reactions have been submitted to VAERS on behalf of at least 84 young girls and 2 boys.[1] Reaction reports have come in from 21 states and the District of Columbia.[2] Reactions were reported for children and young adults ranging in age from 11 to 27. Of the reports indicating what day the vaccine was given and the reaction occurred, 63 percent stated that the reaction occurred the same day the vaccine was given. All but three of the reports were for reactions that occurred within one week of vaccination.
This document is divided into three sections. The first section describes reaction reports for a number of reported adverse events: neurological symptoms including syncopal episodes and seizures, arthralgia and joint pain, Guillian-Barre Syndrome, and other immunological reactions. The second section addresses concerns related to vaccinating individuals already infected with HPV. The last section discusses issues that need to be addressed by government regulators and the manufacturer and considerations for clinicians and consumers.
Reported Adverse Events
Presumably, the reactions described below occurred after the first dose of GARDASIL. GARDASIL is given in a three-dose series. None of the reports stated that the children and adults experiencing problems had previously been vaccinated with GARDASIL.
Syncopal Episodes and Seizures. One-quarter of all reports filed after GARDASIL vaccination were for neurologic adverse events including loss of consciousness, syncope, syncopal events and seizures. An additional five reports included symptoms of dizziness and feeling faint.
Syncope is defined as a temporary suspension of consciousness due to generalized cerebral ischemia (inadequate blood flow and lack of oxygen). The reports of syncopal episodes and their descriptions are remarkable. A physician from Washington State reported that in one morning, three patients experienced syncopal episodes. On August 8th another physician's office reported that two patients experienced syncopal episodes on the same day.
Although these reports did not detail what happened to the individuals experiencing these syncopal episodes, other reports did. The 14-year-old DC girl mentioned earlier experienced a syncopal episode combined with amblyopia (poor vision in one eye), abnormal speech, vomiting, and headache. Also experiencing vision problems, a 17-year-old New York girl reported feeling dizzy and her vision went "black for a few seconds" and she turned pale and lips turned purple and she also had fever and chills. Similar to the DC girl, on July 18th immediately after being vaccinated, a 22-year-old Kentucky woman experienced slurred speech accompanied by pallor and shock. On August 29th, two hours after being vaccinated, a 15-year-old New York girl who had a history of asthma and was on four asthma medications experienced difficulty swallowing prompting a visit to the emergency room. On August 17th, 15 minutes after being vaccinated, a 14-year-old Pennsylvania girl passed out in the car on the way home.
Most of the reports do not describe what happened as a result of the syncopal episode but a few do. One 11-year-old Florida girl fell from the examining table and two Washington girls fell - a 16-year-old girl fell and hit her head on a carpeted concrete surface and a 14-year-old girl fell down and broke her nose.
Whether the 22 girls who experienced syncopal episodes actually experienced atonic seizures cannot be determined from these reports. Four girls, however, displayed observable seizure activity. The 11-year-old Florida girl who fell from the table also displayed "tonic posturing." Tonic posturing is a type of seizure where sustained contraction of muscles in the legs and arms occurs and consciousness is impaired. The 16-year-old Washington girl who fell and hit her head on the floor lost consciousness for one minute and displayed tonic posturing of her right hand. Additionally, a 15-year-old girl from Virginia was described as having "a mild seizure." In California, a 13-year-old girl was walking down the hall after her vaccination, fell and had a 15-second tonic/clonic seizure. Tonic/clonic seizures are also known as "grand mal" seizures.
Additionally, there were reports of dyskinesia (difficulty or distortion in performing voluntary movements) and hypokinesia (slow or diminished movement of the body musculature) both of which have neurological implications.
Arthralgia, Joint Pain and Fever. Arthralgia is defined as pain in the joints. Concerns about arthritis were raised during the GARDASIL clinical trials. Reports of arthralgia in one or more joints accompanied by fever were noted in five instances from four young girls and women in Wisconsin, Texas and New York, and one 18-year-old New York male.
Guillain-Barre Syndrome. Reports state that two recently vaccinated 16-year-old girls - one from Illinois and the other from Mississippi - were diagnosed with Guillian-Barre Syndrome (GBS) following vaccination with GARDASIL. In both cases, the onset of symptoms occurred 13 days after vaccination. According to the National Institute for Neurological Disorders and Stroke:
GBS is a serious disorder in which the body's immune system attacks part of the peripheral nervous system. The first symptoms of this disorder include varying degrees of weakness or tingling sensations in the legs. In many instances, the weakness and abnormal sensations spread to the arms and upper body. These symptoms can increase in intensity until certain muscles cannot be used at all and, when severe, the patient is almost totally paralyzed. … Vaccinations can trigger onset of GBS.[3]
The Illinois girl described earlier was vaccinated on July 7th and symptoms were evident by July 20th. The girl also experienced gait abnormalities (trouble walking properly), asthenia (weakness without loss of strength), paresthesia (burning, prickling, tingling or numbness sensation usually felt in the hands, arms, feet and legs), and hyperkinesia (abnormal increase in muscle movement). The Mississippi girl was vaccinated on July 31st and by August 13th she had increasing numbness and tingling in her feet and hands and was subsequently evaluated by a neurologist and diagnosed with GBS. The current health status of these girls is not known.
In both of these cases, the girls were also vaccinated with Aventis Pasteur's Menactra, a vaccine for meningococcal infections. Menactra has previously been associated with Guillain-Barre Syndrome, and the FDA and others have issued alerts.
Other Adverse Reactions. Additionally, a number of other reactions to GARDASIL are noted in VAERS reports and they include: urticaria (hives); pruritus (itching); macular and papular rashes; blisters and vesicles near the injection site; swollen arms; lymphadenopathy (swollen lymph nodes); red, hot swollen knots at injection site; burning, stabbing, severe and radiating pain at the injection site and in the affected limb during and after injection; nausea and vomiting; infections and skin ulcers, and other allergic reactions.
Other Considerations
GARDASIL is marketed as a "cervical cancer vaccine" and intended to prevent infection with specific HPVs - common viruses among sexually active women. It isn't clear what benefits or potential harms could arise from vaccinating sexually active women who have already contracted HPV. Of the 86 reaction reports filed with VAERS so far, 12 reports were generated by young women 18 and older who were taking hormonal contraceptives and presumably sexually active.
With respect to concerns related to vaccinating women with known HPV infections, adverse reaction reports were filed on behalf of a 17-year-old Texas girl who was already diagnosed with HPV and genital warts. Similarly, the 22 year-old Kentucky woman who experienced slurred speech following vaccination already had an abnormal pap smear with evidence of cervical dysplasia.
Implications
The early reports of potential safety problems with GARDASIL raise concerns and questions that need to be addressed by government regulators, manufacturers and prescribing physicians. Specifically, the following concerns need to be addressed:
- Syncope, seizures and Guillian-Barre Syndrome have now been reported with hours to a week after GARDASIL vaccination. GARDASIL manufacturer, Merck, should add these serious adverse events to the product manufacturer insert.
- Considering that over 20 girls have experienced syncopal episodes sometimes combined with seizures and serious injuries, physicians should consider only giving GARDASIL when the patient is safely laying down on the examining table. Because there seems to be syncopal reactions up until 15 minutes after vaccination, patients should be asked to lie down for 15 minutes after receipt of GARDASIL.
- The information provided by Merck indicates that it is safe to administer GARDASIL with Hepatitis B vaccine. The prescribing information states, "Results for clinical studies indicate that GARDASIL may be administered concomitantly (at a separate injection site) with hepatitis B vaccine (recombinant). Co-administration of GARDASIL with other vaccines has not been studied." [4] Due to the small number of girls aged 9 to 15 who appear to have been evaluated for GARDASIL safety in Merck clinical trials (fewer than 2,000) and lack of publicly available information about how many of these girls were given GARDASIL and hepatitis B vaccine simultaneously, the safety of administering GARDASIL and hepatitis B vaccine to all pre-adolescent girls is uncertain.[5]
- Aside from Hepatitis B, Merck does not state that it is safe to simultaneously administer GARDASIL with any other vaccine. Considering that there are ongoing evaluations of a reported association between Menactra (meningococcal vaccine) and Guillain-Barre Syndrome, and Merck does not explicitly indicate that it is safe to administer to administer GARDASIL and Menactra simultaneously, consumers and clinicians should question whether administering both GARDASIL and Menactra at the same time is safe.
- Similarly, adverse reactions were reported when GARDASIL was administered with eight other vaccines: Hepatitis A, MNQ (?), MEN (Menactra), TD (Tetanus and Diptheria Toxoids), DPP (Diptheria/Pertussis/Polio), PNC Prevnar (Heptavalent pneumococcal conjugate), DTaP (Diphtheria And Tetanus Toxoids and Acellular Pertussis Vaccine), and TDAP (Tetanus, Diptheria and Pertussis). Because Merck does not state that it is safe to administer simultaneously GARDASIL with any vaccine other than Hepatitis B, consumers and clinicians should question whether co-administration of GARDASIL and other vaccines is safe.
- Most, if not all, of the reactions reported to VAERS were in response to the first of the three doses of GARDASIL. The Centers for Disease Control (CDC) Vaccine Information Sheet (VIS) developed for HPV vaccine states that severe reactions include "any unusual condition, such as a high fever or behavior changes. Signs of a serious allergic reaction can include difficulty breathing, hoarseness or wheezing, hives, paleness, weakness, a fast heart beat or dizziness." [6] The CDC also states that "anyone who has ever had a life-threatening allergic reaction to yeast, to any other component of HPV vaccine, or to a previous dose of HPV vaccine should not get the vaccine." Which of the reactions reported to VAERS constitute a "life-threatening allergic reaction" and which, if any, of the children and young adults who experienced reactions should receive additional doses of vaccine? At the October 2006 ACIP meeting, CDC staff stated that only "three serious reports were reported to VAERS after HPV vaccination in females 14 and 16 years of age. One of these patients had vasovagal syncope and was hospitalized overnight for observation." [7]CDC's summary of the first 76 VAERS reports suggests that CDC doesn't regard the remaining reports as "serious." CDC needs to clarify which of the reactions reported to VAERS constitute contraindications to further vaccination with GARDASIL and make this information available to the public and to prescribing physicians.
- What were the short and longer-term outcomes for the individuals who experienced the reactions reported to VAERS? Is there information available that would help to predict the characteristics that predispose one to be at greatest risk of experiencing a serious reaction?
- The CDC's Vaccine Information Sheet indicates that allergy to yeast is a reason to avoid taking GARDASIL. Merck notes that contraindications to the vaccine include "hypersensitivity to the active substances or to any of the excipients of the vaccine. Individuals who develop symptoms indicative of hypersensitivity after receiving a dose of GARDASIL should not receive further doses of GARDASIL." The prescribing information provided by Merck does not specifically note that yeast allergy is a contraindication to taking GARDASIL. Government regulators and the manufacturer need to address the discrepancy between these documents and clarify the issues related to yeast allergy and make this information readily available to the public and prescribing physicians.
- Additionally, Merck notes that vaccine ingredients include 225 mcg of aluminum (as amorphous aluminum hydroxyphosphate sulfate adjuvant), 0.78 mg of L-histidine, 50 mcg of polysorbate 80, and 35 mcg of sodium borate. These ingredients are not listed on the CDC's VIS sheet. The public needs this information so that they can identify whether they have "hypersensitivities" to any of the ingredients and whether they are at risk of experiencing a serious allergic reaction. Hypersensitivities and known allergic reactions are critical pieces of information that need to be communicated to prescribing physicians in order to make the safest possible vaccination decisions.
Government regulators including the CDC and FDA, in combination with Merck, should address the above safety concerns as soon as possible. Medical groups advocating use of GARDASIL should effectively communicate to physicians and patients the potential risks of using GARDASIL along with precautions to improve the safety of patient care.
For more information about HPV infection, GARDASIL vaccine and VAERS adverse event reports pertaining to GARDASIL, go to www.NVIC.org
[1] National Vaccine Information Center. VAERS reports related to HPV4 vaccine http://www.medalerts.org/vaersdb/findfield.php?PAGENO=2&PERPAGE=10&VAX=HPV4 (Accessed January 10, 2007).
[2] States reporting adverse reactions include: Arkansas (1), Arizona (3), California (14), Colorado (1), District of Columbia (1), Florida (4), Georgia (1), Illinois (2), Kansas (1), Kentucky (3), Massachusetts (4), Maine (1), Missouri (1), North Carolina (4), New Jersey (2), New York (7), Pennsylvania (4), Rhode Island (3), Tennessee (1), Texas (9), Virginia (1) and Washington (4). State of residence was not identified in at least 14 cases.
[3] National Institute of Neurological Disorders and Stroke. Guillain-Barre Fact Sheet http://www.ninds.nih.gov/disorders/gbs/detail_gbs.htm. Accessed January 31, 2007.
[4 ] Merck and Co., Inc. GARDASIL. www.merck.com/product/usa/pi_circulars/g/GARDASIL/GARDASIL_pi.pdf (Accessed January 31, 2007).
[5] Food and Drug Administration. Product Approval Information – Licensing Action: GARDASIL Questions and Answers. http://www.fda.gov/cber/products/hpvmer060806qa.htm. Study 016: Studies in Younger Age Groups, page 19; Study 018: Safety and immune response younger children, page 20.
[6]Centers for Disease Control, National Immunization Program, HPV Vaccine Information Sheet www.cdc.gov/nip/publications/VIS/vis-hpv.pdf (Accessed January 31, 2007.)
[7]Centers for Disease Contol, National Immunization Program, ACIP Meetings October 2006 http://www.cdc.gov/nip/ACIP/minutes.htm (Accessed January 31, 2007.)
Survey of HPV Vaccine (Gardasil) Cost
NVIC Pivate Pediatrician Office Survey
Northern Virginia Area HPV Vaccine (GARDASIL) Costs
January 2007
In January, 2007 NVIC conducted a small survey of private pediatrician offices in the Washington, D.C. area. Four pediatric practices in the Northern Virginia area were contacted to determine the total cost of the HPV three-dose series, administration fees and office visit charges.
The cost for GARDASIL vaccine and administration fees ranged from $420 to $825 for a three-dose series plus additional charges for each office visit to receive the shot. The additional charges for the three office visits related to administration of the vaccine varied depending upon if the patient
(1) is a current patient;
(2) is a new patient;
(3) the vaccine is administered by a physician;
(4) the vaccine is administered by a nurse;
(5) the visit is for a well-child and preventive care;
(6) the visit is for sick child care.
Based on this information, total costs for the series of three GARDASIL vaccinations, related administration fees and office visit charges are estimated to range from $500 to $900 or more.
Pediatric Practice #1
Contacted 1/31/07
GARDASIL Vaccine and Administration Fee: $420 ($140 per shot)
Office Visit Charge: $150 to $185 for new patient or no charge to $88 for current patient.
Three Shot Series: Minimum $420 plus office visit charges.
Pediatric Practice #2
Contacted 1/31/07
GARDASIL Vaccine and Administration Fee: $555 ($185 per shot)
Office Visit Charge: $110-205 for new patient, less for current patient.
Three Shot Series: Minimum $555 plus office visit charges.
Pedatric Practice #3
Contacted 1/7/07
GARDASIL Vaccine and administration fee: $525 ($175 per shot)
Office visit charge: $35 to $83
Three Shot Series: Minimum $630
Pedatric Practice #4
Contacted 1/31/07
GARDASIL Vaccine and Administration Fee: $825 ($275 per shot)
Office Visit charge: $82 for new patient, less for current patient.
Three Shot Series: Minimum $825 plus office visit charges.
Human Papilloma Virus Vaccine Safety
Analysis of Vaccine Adverse Events Reporting System Reports Part II
Human Papilloma Virus Vaccine Safety
Analysis of Vaccine Adverse Events Reporting System Reports Part II
by Vicky Debold, PhD, RN and Barbara Loe Fisher
February 21, 2007
This document is a follow-up to the February 1, 2007 report detailing adverse events, concerns and clinical implications related to use of GARDASIL based on submissions made to the federal Vaccine Adverse Event Reporting System (VAERS). This report covers all reactions submitted in 2006 and supplements the first NVIC analysis of reported adverse events - NVIC Analysis of VAERS Reports on GARDASIL - which can be found here. Readers are encouraged to review the previous report. A number of clinical and patient safety concerns and potential problems arising from co-administration of GARDASIL with other vaccines not evaluated by the manufacturer were discussed in the previous report.
Also, readers are referred to the VAERS website for cautions related to use of VAERS data.[1] The information in this report does not prove a cause and effect relationship between any of the reactions and administration of GARDASIL. This document only summarizes the information in VAERS as it relates to use of GARDASIL, either alone or in combination with other vaccines.
[2]
As of mid-February 2007, there were 430 case reports associated with administration of the GARDASIL vaccine. After the duplicate case records were removed (about 10 percent of files), 385 unique case records remained. The data from the 385 case reports are used for this analysis unless stated otherwise.
Since GARDASIL was licensed on June 8, 2006 and added to the recommended American vaccination schedule on June 29, 2006, the number of reports per month filed with VAERS has steadily increased. Two reports were filed in July, 12 in August, 65 in September, 79 in October, 108 in November and 119 in December. Between June 8 and December 31st, an average of 1.87 [3] reports per day have been filed. In December, 3.97 reports per day were filed.
As previously reported, the earliest adverse event report date following vaccine licensure and recommendations was July 14, 2006 for a girl vaccinated July 7, 2006. The earliest reported adverse event overall was for a woman vaccinated on September 1, 2003 during a clinical trial. The most recent report included in this analysis was submitted December 28, 2006.
Reports were submitted to VAERS at various dates throughout each month but tended to be grouped around four dates. In particular, 247 reports (64 percent of all reports) were submitted on September 19th (44 reports), October 17th (45 reports), November 16th (108 reports) and December 18th (87 reports).
Patient Demographics
In 98 case reports (25.4 percent) age was not identified. Patient age, where reported, ranged from 1 to 57 years. Among the case reports where age was identified, 111 reports (38.7 percent) were submitted for children 16 and under. The distribution by age of these children is as follows: 1.0/1.5 years (2 cases)[4], 9 (1 case), 11 (9 cases), 12 (10 cases), 13 (14 cases), 14 (25 cases), 15 (27 cases) and 16 (23 cases).
In 14 reports, the gender of the patient was not identified. In all but four of the remaining cases, the patients were females. The males for whom case reports were submitted were 1.0, 1.5, 12 and 18-years-old.
As of the end of December, case reports have been submitted to VAERS from 43 states and the District of Columbia. Two months earlier, reports had been submitted from 20 states.
Description Of Reported Reactions
In the case reports submitted to VAERS, five of the reactions were described as "life-threatening," six were "disabling," and 210 (54.5 percent) had "not recovered" as of the date data were provided by VAERS. Hospitalization was reported in 12 cases and two-thirds sought additional care in an emergency room or doctor's office (see Use of Health Services section).
A number of symptoms were reported, with varying levels of severity.[5] The most frequently reported reactions were: pain of various types (89), syncope (55) and dizziness (41), fever/pyrexia (41), paresthesia and hypoaestesia (32), rash (33) and pruritis/itching (31), vasodilation (19), headache (19), and vomiting (16). The following sections focus on three reactions: syncope and dizziness, paresthesia, and Guillain-Barre Syndrome.
Descriptions of the symptoms presented in the following condition-specific sections includes verbatim passages taken from VAERS case reports. No attempt was made to correct spelling or modify what was reported. Symptom description passages are often truncated mid-sentence in the VAERS database.
The time of reaction onset was noted in 192 reports (72 percent). Among the reports that noted when the reaction occurred, 60 percent (115 reports) indicated a reaction on the day the vaccine was given. An additional 21 percent (41 reports) stated that the reaction occurred the day after the vaccine was given. Reports indicated that 94 percent (181 reports) occurred within one week of vaccination.
Syncope
Syncope is defined as a temporary suspension of consciousness due to generalized cerebral ischemia (inadequate blood flow and lack of oxygen).
At the end of December 2006, there were 62 case reports stating that a patient had experienced a syncopal episode suggesting that 14.4 percent of case records involve syncope and/or fainting (62/430).[6] Among children 9 to 16 years of age, 12.2 percent of case reports (14/115) documented a syncopal episode. In comparison, only 5.8 percent of children 9 to 16 years of age who received the Tdap (tetanus-diptheria-pertussis) vaccine experienced a syncopal episode.[7]
When syncope is considered along with reports of dizziness (41 cases), approximately 25 percent of all VAERS case reports are involved. Although most syncope reports indicate that it occurred immediately after vaccine administration, some do not. Despite the impression that syncopal episodes are commonplace and harmless, some of the episodes appear to be atypical and worthy of attention. Specifically of concern are the reactions that occur well after the vaccine was given and, in some cases, are combined with seizures and injuries because the patients were either in a sitting or standing position when the syncopal event occurred, fell, and suffered injuries including fractures as a result. For example:
- Case record - 269192 - (14 y/o) - Information has been received from a physician concerning a 14 year old female who on an unspecified date was vaccinated with Gardasil (yeast). It was reported that the patient was sitting on a bench and when the personnel left the room, the patient apparently fainted and ended up falling off bench. It was reported that it was unsure if the patient had broken her nose but there was blood. At the time of this report, the outcome of the events were unknown. The physician feels that the patient "might be anore [report truncated]
- Case record - 260144 - (13 y/o) - Patient received Hep A in right arm. Then received HPV in left arm. C/O pains, numbness. Started walking down hall fainted and had tonic/clonic movement for 15 sec. Symptoms: Dyskinesia, hypertonia, hypotonia, pain, paresthesia, syncope
- Case record - 265305 - (21 y/o) - According to my daughter, the following happened: 1. Shot was given between 1100-1115 am 2. Proceed to wait a few minutes with doctor in room and then went to check out 3. Between 1120-1125 am...she passed out in doctors office. 4. Day 2-4: tightness in muscles especially around lower legs. Also tigtness in arms, but not as bad as legs. 5. Hard time walking after first waking up in morning and gradually loosens up after she starts moving around. 6. I suggested to her to take a Tylenol to help with m [report truncated]. Symptoms: Gait abnorm, hypertonia, syncope.
- Case record - 262242 - (14 y/o) - Vasovagal syncope shortly after receiving hep A and Gardasil vaccine, fell, hit nose on a drawer, loss of consciousness, sent to ER in transport broke nose. Symptoms: Bone fract spontan, injury accid, syncope.
- Case record - 264959 - (14 y/o) - Administr4ed Vaqta, then Gardasil, immediately after Gardasil child became unresponsive, stiff, pale about 30 sex. Child lost bladder control, pulse 46-55 after event, child alert and aware after events. Symptoms: Arrhythmia, bradycardia, hypertonia, incontin urin, pallor, stupor, syncope.
NVIC again raises questions about the advisability of allowing children who are believed to be susceptible to syncopal episodes to be vaccinated in a sitting position, left unattended, and not required to lie down for at least 5 to 15 minutes following vaccination.
Paresthesia [8]
Paresthesia (paraesthesia) is an abnormal sensation of the skin, such as numbness, tingling, pricking, burning, or creeping on the skin that has no objective cause. Hypesthesia (hypoaesthesia) is the name of the condition that occurs when the skin loses some of its sensitivity to pain or touch. Both have a number of causes and are symptoms of a number of both minor and serious diseases and conditions.
As of the end of December 2006, there were 34 case reports stating that patients had experienced either paresthesia, paraesthesia or hypoaesthesia episodes. Thus, 7.9 percent of case records involve reports of paresthesia-type symptoms (34/430).[9] Among children 9 to 16 years of age, 7.0 percent of case reports (8/115) documented a paresthesia episode. In comparison, only 1.6 percent of children 9 to 16 years of age who received the Tdap vaccine (tetanus-diptheria-pertussis) experienced paresthesia symptoms.
- Case record - 264761 - (18 y/o) - Information has been received from a physician concerning his "around 18" year old daughter who was vaccinated IM with a 0.5 ml dose of HPV rL1 6 11 16 18 VLP vaccine (yeast). Concomitant therapy included a dose of diphtheria toxoid (+) pertussis acellular 3-component vaccine (+) tetanus toxoid (BOOSTRIX). Subsequently, the patient experienced numbness in her arms and lower extremities. Unspecified medical attention was sought. On an unspecified date, the patient recovered. No product quality complaint was [report truncated]. Symptoms: Hypesthesia.
- Case record - 264950 - (57 y/o) - 09/19/2006 MMR and HBV adm 1, 10/03/2006 swollen glands, numbness and tingling of arm and legs bilat. Pt called hotline and states she was told this was from the MMR. 10/15/06 chest pain. Pt wonders if this is related to immunizations. Symptoms: Lymphadenopathy, chest pain, paresthesia.
- Case record - 267171 - (13 y/o) - INfluenza vaccine and Human Papilloma VIrus vaccines given November 10 about 11:45 AM. Patient woke up with numbness on right side of face on November 11. Presented to Emergency Room on November 12 with Bells Palsy of right side of face. Unable to move right side of face. Unable to close right eye. Previously healthy, no symptoms on day of vaccine administration. Symptoms: Paralysis, facial paralysis, paresthesia.
- Case record - 268459 - (14 y/o) - With in five minutes of administration complaint of headache, then felt faint. I had my daughter sit down in the waiting room with her head down before we walked out. Not thinking this had anything to do with the vaccine. We walked out the door and she began have difficlty walking, weaving, I asked her if she was okay and she could only respond yes. I took her arm and tried to turn her back to the Doctor's office and she collapsed on the ground. Though, she remainded conscious her eyes were glazed and [record truncated]. Symptoms: Cold sweat, difficulty in walking, disorientation, dizziness, dyskinesia, headache, hyperventilation, pallor, paraesthesia, tinnitus, tremor
Guillain-Barre Syndrome
According to the National Institute for Neurological Disorders and Stroke:
As of the end of December 2006, there were five case reports that identified Guillain-Barre Syndrome in GARDASIL recipients. The first two of the case reports were described in NVIC's first report. As previously stated, both girls had also received a meningococcal vaccine at the same time that they received GARDASIL.[10]
The three new case reports involve two 13-year-old girls, from Arkansas and Washington, and a 16-year-old from Ohio. All three of these girls received only GARDASIL.
- Case report - 265839 - (16 y/o) - Information has been received from a physician concerning a 16 year old female who on an unspecified date was vaccinated with the first dose of HPV vaccine. subsequently the pt developed lower extremity weakness and was hospitalized for two days with Guillain Barre. The pt received treatment with gamma globulin during her hospitalization. At the time of this report, the pt was back to school and was 99% recovered having only some area of numbness to her lower extremities. The physician did not believe that [report truncated]. Symptoms were: asthenia, Guillain-Barre Syndrome, and paresthesia.
- Case report - 268143 - (13 y/o) - Pt admitted to hospital on 12/1/06 with chief complaint of ascending weakness bilaterally, upper and lower extremities. Neuro consult diagnosed Guillain-Barre syndrome. Pt received the Gardasil vaccine on 11/22/06. Resident MD asked pharmacist to write up possible ADR of Guillain-Barre from this vaccine. Symptoms: CSF test abnormal, Guillain-Barre Syndrome, muscular weakness
- Case report - 269328 - (13 y/o) - Information has been received from a physician concerning a 13 year old female who on 22-Nov-2006 was vaccinated with HPV rL1 6 11 16 18 VLP vaccine (yeast). A few days later she had cold symptoms and was prescribed azithromycin (ZITHROMAX). A few days after that the patient developed hives and was prescribed amoxicillin. A few days later the patient was hospitalized and diagnosed on 01-Dec-2006 with Guillian-Barre Syndrome. The patient sought unspecified medical attention. At the time of the report, it was [reported truncated] Symptoms: Guillain-Barre Syndrome, urticaria (hives).
Human Papilloma Virus Infections
There were several case reports of infection with human papilloma virus and cervical dysplasia and genital warts. For example, case reports 260907, 267457, and 269228 describe infections with genital warts, and 267410, 267480, 269248 reported infection with HPV; and 263204, 267415, 268483, 269192, 269202, 269213 report abnormal pap smears and in some cases, cervical dysplasia. A few of the case reports note that procedures to address dysplasia were planned including colposcopy and loop electrosurgical excision procedures.
In most cases, it was not documented whether patients were infected prior to vaccination and whether an exacerbation of disease was experienced following vaccination. Two exceptions include:
- Case report - 267457 - (age not reported) - Information has been received from a midwife registered nurse concerning a female with genital warts who "within the last few weeks" was vaccinated with HPV rL1 6 11 16 18 VLP vaccine (yeast). Concomitant therapy included imiquimod (Aldara). Subsequently "within the last few weeks" the patient experienced "swelling in genital warts". At the time of the report the patient was recovering. Unspecified medical attention was sought. Other information has been requested. Symptoms: Edema
- Case report - 267418 - (22 y/o) - Information has been received spontaneously from a gynecologist concerning a 22 year old female patient who had participated in an HPV study. On 29-SEP-2006 the patient tested positive for high risk HPV. The patient's outcome was unknown. Upon internal request, the patient was unblinded on 25-OCT-2006, she received her first dose of HPV on 06-FEB-2003, her second dose in March 2003, and her third dose in September 2003. She showed strong conversion to all 4 vaccine types. At baseline the patient had been se…[report truncated]. Diagnostic lab data - 07-DEC-2004: "ASC-H". 04-APR-2005 colposcopy biopsy negative for lesion, discrepancy noted - biopsy was of less severity than the Papanicolaou (PAP) test result and repeat colposcopy, PAP, and endocervical curettage (ECC) (among other choic…[report truncated]. Symptoms: No drug effect
Co-administration of Vaccines
The manufacturer's insert only evaluated co-administration of GARDASIL with Hepatitis B vaccine. The case reports submitted to VAERS indicate that GARDASIL has been administered simultaneously with 18 additional vaccines. As categorized by VAERS, the following vaccines were given along with GARDASIL: DPP, DTAP, DTOX, FLU, FLUN, HEPA, HEPAB, HEP, IPV, MEN, MMR, MNQ, TDAP, TTOX, VARCEL, HIB, MMRV, PNC, and TD. The case reports show that 42 patients (13.9 percent) received at least one other vaccine along with GARDASIL. Additionally, 13 patients received three vaccines, three received four vaccines, and one, the 1-year-old infant, received five vaccines. In only two case reports was Hepatitis B the additional vaccine simultaneously administered. Among those who received two or more vaccines, 68.3 percent were 16 or younger. Of those 16 or younger for whom an adverse event report was submitted, 25.2 percent had received two or more vaccines.
Use Of Health Services Among Patients Reporting A Reaction
The case reports indicate that in 253 instances (66 percent), additional ambulatory services were used. It is not possible to determine how many total additional visits were made or to distinguish how many of the additional visits were made to an emergency room rather than a physician's office. Of those requiring additional visits, 57 (22.5 percent) were made by children 15 and under. Among all children 15 and under, 64.8 percent required an additional emergency room or doctor's office visit.
Case reports indicate that 12 patients ranging in age from 13 to 23 were admitted to the hospital and stayed up to five days. Table 1 reports the age and state of residence of the patient. It also shows the number of days following vaccination, use of vaccines other than GARDASIL, symptoms, narrative description and use of tests as noted in the VAERS database.
Concerns
NVIC previously raised concerns about the seriousness of some of the reported reactions. Syncopal episodes combined with seizures and injury, as well as certain types of paresthesia, weakness, paralysis and Guillain-Barre Syndrome are serious health events. In the interest of protecting the public's health, action is needed on the part of all stakeholders.
Doctors, nurses and parents should promptly report all reactions following vaccination to VAERS. Failure to submit reports of all reactions to VAERS weakens efforts to monitor and improve the safety and effectiveness of the national vaccination program.
Doctors, nurses and parents should note that a substantial number of children are experiencing dizziness and syncopal episodes after vaccination and some are sustaining injuries when they lose consciousness and fall. This is a clear patient safety issue and children should be protected from potential and unnecessary injury whenever possible. They should be vaccinated laying down and required to lie down for at least 5 to 15 minutes after vaccination. They should not be left unattended.
The manufacturer's product insert should be updated to include explicit precautions related to use of GARDASIL in combination with vaccines not evaluated during clinical trials. Nearly 25 percent of children were vaccinated with one or more of 18 vaccines not evaluated by the manufacturer. There are no evidence-based guidelines that support use of such a protocol. NVIC is also concerned that the numbers of children vaccinated with Hepatitis B along with GARDASIL during the clinical trials were insufficient to establish safety. Until the adverse reactions reported to VAERS can be evaluated fully, doctors and nurses should not administer other vaccines at the same time GARDASIL is given.
The manufacturer's product insert should also be updated to include the serious reactions that have been reported to VAERS.
[1] Information about the VAERS reporting system can be found here http://vaers.hhs.gov. The government has issued the following warning to people using VAERS data: When evaluating data from VAERS, it is important to note that for any reported event, no cause and effect relationship has been established. VAERS is interested in all potential associations between vaccines and adverse events. Therefore, VAERS collects data on any adverse event following vaccination, be it coincidental or truly caused by a vaccine. The report of an adverse event to VAERS is not documentation that a vaccine caused the event.
[2] Unless otherwise noted, our report uses the data for adverse events reported July 1, 2006 to November 30, 2006 and retrieved on two dates in January and February 2007. For the adverse events reported December 1 through December 31, 2006, our report uses data retrieved mid-February 2007. As of February 18, 2007 some of the data in the VAERS database – reported symptoms, in particular -- have been modified. See footnote related to paresthesia.
[3] Between recommendation date (6/29/07) and December 31, 2007, 2.07 reports per day have been filed with VAERS. Per day reports by month: July .06, August .39, September 2.63, October 2.55, November 3.60, and December 3.97.
[4] In two cases, GARDASIL was unintentionally given to two male infants.
[5] Symptoms reported are as follows (in alphabetical order): acne (3), abasia (2), abdominal pain (3), abnormal behaviour (1), abortion (1), agitation (3), albuminuria (1), allerg react (3), amblyopia (3), amenorrhea (1), angioedema (1), anorexia (1), anxiety (2), applicat site react (2), arrhythmia (3), arthralgia (7), asthenia (14), asthma (1), bacterial infection (1), blood glucose increased (1), blood potassium decreased (2), blood pressure decreased (1), blood pressure increased (1), blood urine present (1), body temperature decreased (1), bone fract spontan (1), bradycardia (2), breast pain (1), cellulitis (2), chest pain (3), chills (7), cold sweat (1), colitis (1), condition aggravated (2), confusion (2), conjunctivitis (1), contusion (2), convulsion (5), convulsion grand mal (1), cough inc (1), csf test abnormal (1), culture urine positive (1), cyanosis (1), decreased appetite (2), dehydration (3), depersonal (1), depression (1), diarrhea (2), difficulty in walking (1), diplopia (1), discomfort (1), disorientation (1), dizziness (41), dry eye (1), dyskinesia (2), dysphagia (1), dyspnea/ dyspnoea (7), dysuria (1), ecchymosis (1), edema/oedema (9), edema face (8), edema inject site (4), edema/oedema periph (8), edema tongue (1), emotion labil (1), epistaxis (1), erythema (2), esr inc (1), euphoric mood (1), eyes gaze upward (1), fall (1), fatigue (1), feeling abnormal (1), fever (36), flu synd (4), flushing (1), fracture (1), gait abnorm/disturbance (3), guillain barre synd (5), haemorrhage (1), headache (19), hem (1), hepatitis (2), herpes simplex (2), high-pitched crying (1), hostility (1), hyperhidrosis (5), hyperkinesia (3), hypersensitivity (1), hypertonia (9), hyperventil (2), hypesthesia (5), hypoaesthesia (4), hypokinesia (4), hypotens (1), hypotonia (1), hypoxia (1), hysn inject site (5), incontin urin (1), incorrect route of drug administration (1), infect (2), infect fung (2), infect viral (2), inflam inject site (1), influenza like illness (5), inject site react (13), injection site anaesthesia (1), injection site erythema (4), injection site induration (1), injection site mass (9), injection site oedema (2), injection site pain (5), injection site rash (2), injection site swelling (1), injury accid (5), insomnia (2), joint stiffness (1), lab test abnorm (7), lactation fem (1), laryngismus (3), loss of consciousness (6), lymphadenopathy (10), malaise (12), med error (9), menorrhagia (1), menstrual disorder (1), menstruation irregular (1), migraine (2), movement dis (1), mucous mem dis (1), muscular weakness (2), myalgia (13), mydriasis (1), myositis (1), nausea (30), neopl skin (1), nervousness (1), no drug effect (1), nodule skin (1), pain (38), pain abdo (5), pain back (1), pain chest (2), pain inject site (37), pain pelvic (1), pallor (9), paralysis (1), paralysis facial (3), paresthesia/paraesthesia (28), pelvic pain (1), pharyngitis (2), photosensitivity (1), platelet count decreased (1), post vac synd (2), pregn unintend (1), pregnancy test false positive (1), pregnancy test negative (1), pruritus (31), pruritus genital (1), pyrexia (5), rash (26). rash erythematous (1), rash mac pap (5), rash macular (1), rash vesic bull (5), react aggrav (2) react uneval (4), rhinitis (1), rhinorrhoea (1), scan brain (1), screaming synd (1), sensation of heaviness (3), shock (1), similar reaction on previous exposure to drug (1), skin discolor (2), skin disorder (1), skin papilloma (1), smear cervix abnormal (2), spasm general (2), speech dis (2), stomatitis ulcer (1), stupor (2), suicide attempt (1), sweat (5), swelling (2), syncope (55), taste pervers (1), thinking abnorm (1), tinnitus (2), tremor (3), twitch (2), ulcer skin (2), unintended pregnancy (1), urticaria (23), vaccination complication (1), vaginitis (2), vasc dis periph (1), vasodilat (19), vertigo (1), viral infection (2), vision abnorm (1), vision blurred (2), visual disturbance (1), vomit (16), wheezing (1), white blood cell count increased (1), white blood cells urine positive (1).
[6] Computations in this section used VAERS data that contained duplicate entries. NVIC found duplicate records in the VAERS database, including among case reports of syncope. After correcting for duplicate records, 14.3 percent of patients (55/385) experienced a syncopal episode.
[7] At the same office visit, three children also received Hepatitis A vaccine and one received chickenpox vaccine.
[8] When the VAERS database was checked on two different dates in February 2007, in a number of cases the person reporting the adverse event to VAERS (e.g., a doctor, nurse, or parent) included the symptom paresthesia. However, in the current version of the VAERS database, a number of these earlier reports have been modified without explanation. Either the symptom was removed entirely or re-spelled as “paraesthesia” or more frequently, reclassified as hypoaesthesia. For the this analysis, the three terms were grouped.
[9] Computations in this section used uncorrected VAERS data that contained duplicate entries. Using data corrected for duplicate entries, the rate for paresthesia-type symptoms was 8.3 percent (32/385).
[10] National Institute of Neurological Disorders and Stroke. Guillain-Barre Fact Sheet http://www.ninds.nih.gov/disorders/gbs/detail_gbs.htm. Accessed January 31, 2007.
[11] Data in Table 1 are taken verbatim from the VAERS database. The narratives within case reports are frequently truncated mid-sentence. Additionally, data for age, date of vaccination and interval between vaccination and onset of symptoms is sometimes missing.Gardasil Rally Statement
Barbara Loe Fisher, Co-Founder and President March 8, 2007, Washington, D.C.
STATEMENT
Barbara Loe Fisher, Co-Founder and President
National Vaccine Information Center
March 8, 2007, Washington, D.C. Rally sponsored by
Parents and Citizens Committee to Stop Medical Experimentation in D.C.
Just 15 days after the Centers for Disease Control recommended that all 11 and 12 year old girls in America get three doses of Merck’s HPV vaccine, GARDASIL, the very first GARDASIL reaction report was filed with the federal Vaccine Adverse Event Reporting system. The report described a 14 year old District of Columbia girl, who was injected with GARDASIL on July 11, 2006 and complained of severe pain at the injection site, lost consciousness, fell off the examining table, regained consciousness and experienced tingling, numbness, pain and twitching in her hands and feet, headache and blurry vision. She vomited in the parking lot, lost her speech and was sent to the Emergency Room, where she continued to exhibit difficulty speaking upon neurological examination. This first GARDASIL reaction report, which included collapse and loss of sensation in the hands and feet along with other neurological signs, was a prophetic warning. In the seven months following licensure of GARDASIL, many of the more than 600 vaccine reaction reports involve similar kinds of serious neurological symptoms among young girls and women.
The National Vaccine Information Center joins with the Parents and Citizens Committee to Stop Medical Experimentation in D.C. in calling for public hearings and citizen testimony about whether 11 year old girls living in the nation’s Capitol should be required by law to get three doses of HPV vaccine in order to attend sixth grade. This call for more citizen involvement in new vaccine laws is taking place in every state where HPV vaccine mandates are being aggressively pursued by drug company lobbyists and legislators trying to force young girls to use HPV vaccine without the voluntary, informed consent of parents.
Since 1982 the National Vaccine Information Center has defended the right of parents to have full information about the benefits and risks of vaccines and be allowed to exercise informed consent to vaccination. We oppose HPV vaccine mandates for three reasons.
First, there is a serious absence of scientific evidence that Merck’s GARDASIL is safe to give to young girls entering puberty, who are biologically different from older women. Vaccine mandates target 11 year old girls but Merck only studied the safety of GARDASIL in a few hundred eleven year old girls and followed them up for a few years. Already, there have been more than 600 reports made to the federal Vaccine Adverse Event Reporting System documenting that young girls are collapsing and suffering seizures, loss of sensation in the hands and feet, vision and speech problems, Guillain Barre syndrome, facial paralysis, and other serious neurological symptoms. Merck also admits in its product insert that GARDASIL was not studied for the ability to affect female fertility, cause other kinds of cancer or be toxic to the genes.
Second, there is little or no scientific evidence that Merck’s GARDASIL will actually protect 11 year old girls from getting cervical cancer. The vaccine only contains two high risk HPV types out of 15 types associated with cervical cancer. Officials at the Food and Drug Administration and Centers for Disease Control have expressed concern that the 13 other high risk HPV types could replace the two types in GARDASIL so that HPV associated cervical cancer may not, in fact, be eliminated with widespread use of the vaccine. Young girls may think they can skip routine pap smears because the vaccine protects them from getting cervical cancer when that may not be true at all.
Third, more than 90 percent of all girls and boys, who become infected with HPV, asymptomatically clear the infection from the body. It takes many years of chronic infection for undiagnosed pre-cancerous cervical lesions to progress to cervical cancer, usually because the women have not had routine cancer -detecting pap smears. Cervical cancer has dropped by more than 74 percent in the US since pap smears became part of routine health care for women and today less than one percent of all cancer deaths and newly diagnosed cancers are due to cervical cancer in America.
The more than $4 billion dollars it will take to vaccinate every 11 year old girl in Washington, D.C. and every other state could be better spent on research to find out why so many highly vaccinated children in Washington, D.C. and other public school systems around the nation are chronically ill and disabled, with 1 in 150 autistic, one in 6 learning delayed and millions more asthmatic and diabetic.
In conclusion, HPV vaccine mandates are unnecessary, expensive and potentially dangerous because so little is known about the long term health risks and effectiveness of Merck’s GARDASIL vaccine. Elementary schools should be centers for learning and not centers for forced use of potentially unsafe and ineffective vaccines. The District of Columbia should hold open public hearings and allow parents to present testimony about why they want to become fully informed about the benefits and risks of HPV vaccine and be allowed to make a voluntary vaccination decision for their daughters.
Gardasil & Menactra Adverse Event Reports to the Vaccine Adverse Events Reporting System (VAERS)
An Analysis by the National Vaccine Information Center of
Gardasil & Menactra Adverse Event Reports to the
Vaccine Adverse Events Reporting System (VAERS)
February 2009
The centralized federal Vaccine Adverse Events Reporting System (VAERS) was created under the National Childhood Vaccine Injury Act of 1986 and became operational in 1990. Although there was a mandate in the 1986 law for vaccine providers to report serious health problems, hospitalizations, injuries and deaths to VAERS, there were no sanctions for failure to report.
It is estimated that only between 1 and 10 percent of all adverse health outcomes which occur following vaccination are reported to VAERS. The following information was produced from analyzing VAERS data published by CDC and FDA and made available on the www.NVIC.org website through the MedAlerts searchable VAERS database at www.MedAlerts.org
(through November 30, 2008)
Menactra (meningococcal) vaccine was licensed by the FDA on January 14, 2005 for children and adults aged 11 to 55 years. One dose is recommended by the CDC for boys and girls 11-12 years old and many high schools and colleges are requiring it for attendance. Menactra is routinely administered by pediatricians and family practice physicians to grade school, high school and college students. Between 1,000 and 2,600 cases of meningococcal disease occurs in the U.S. every year and Menactra contains four of the most common strains of meningococcal.
Gardasil (Human Papillomavirus Quadrivalent) vaccine was licensed by the FDA on June 8, 2006 for girls and women aged 9 to 26 years. Three doses are recommended by the CDC for universal use in girls 11-12 years old and Gardasil is routinely administered by pediatricians, obstetrician/gynecologists and family practice physicians to grade school, high school and college students. Chronic human papillamovirus infection is associated with the development of cervical cancer and Gardasil contains four HPV types associated with this sexually transmitted infection.
What follows is a comparison of some of the more serious Gardasil and Menactra adverse event reports submitted to VAERS.
|
SUMMARY STATISTICS |
Gardasil (HPV) |
Comment on Gardasil Reports |
Menactra |
Comment on Menactra Reports |
|
Total Reports |
10,151 |
|
4,436 |
|
|
Life Threatening |
152 |
|
57 |
(18 with HPV) |
|
Emergency Room |
5,021 |
|
1,667 |
|
|
Hospitalization |
458 |
|
268 |
|
|
Did Not Recover |
2,017 |
|
393 |
|
|
Disabled |
261 |
|
29 |
(13 with HPV) |
|
Died |
29 |
(25 HPV alone) |
6 |
(none with HPV) |
|
|
||||
|
SYMPTOM STATISTICS |
|
|
|
|
|
Alopecia |
51 |
(41 HPV alone) |
12 |
(8 with HPV) |
|
Arrhythmia |
18 |
(14 HPV alone) |
4 |
(1 with HPV) |
|
Arthralgia |
276 |
|
119 |
|
|
Arthritis |
68 |
(57 HPV alone) |
18 |
(6 with HPV) |
|
Blindness |
36 |
(31 HPV alone) |
10 |
(2 with HPV) |
|
Blood clot |
23 |
(all HPV alone) |
0 |
|
|
Cardiac arrest |
9 |
(all HPV alone) |
2 |
(none with HPV) |
|
Chest pain |
123 |
|
63 |
(16 with HPV) |
|
Collapse |
44 |
(29 HPV alone) |
18 |
(7 with HPV) |
|
Deafness |
17 |
(11 HPV alone) |
5 |
(4 with HPV) |
|
Demyelination |
12 |
(10 HPV alone) |
10 |
(1 with HPV) |
|
Dizziness |
1,320 |
|
589 |
|
|
Encephalomyelitis |
8 |
(5 HPV alone) |
9 |
(1 with HPV) |
|
Fainting |
278 |
|
41 |
(28 with HPV) |
|
Hair Loss |
36 |
(30 HPV alone) |
8 |
(5 with HPV) |
|
Hemorrhage |
9 |
(7 HPV alone) |
2 |
(2 with HPV) |
|
Joint pain |
126 |
|
37 |
(7 with HPV) |
|
Lupus |
28 |
(27 HPV alone) |
6 |
(1 with HPV) |
|
Migraine |
159 |
|
36 |
(9 with HPV) |
|
Multiple sclerosis |
16 |
(12 HPV alone) |
7 |
(2 with HPV) |
|
Numbness |
351 |
|
149 |
|
|
Pain |
2,422 |
|
1,320 |
|
|
Paralysis |
70 |
(54 HPV alone) |
26 |
(6 with HPV) |
|
Rash |
963 |
|
473 |
|
|
Rheumatoid arthritis |
31 |
(25 HPV alone) |
6 |
(4 with HPV) |
|
Seizures |
544 |
|
158 |
(73 with HPV) |
|
Stroke |
16 |
(all HPV alone) |
1 |
(none with HPV) |
|
Syncope |
1,643 |
|
427 |
|
|
Thrombosis |
34 |
(all HPV alone) |
1 |
(none with HPV) |
|
Tingling |
278 |
|
132 |
|
|
Vasculitis |
11 |
(all HPV alone) |
2 |
(none with HPV) |
|
Rechallenge |
275 |
|
8 |
(7 with HPV) |
Red Flags:
VAERS is a sentinel reporting system, designed to raise “red flags” for unusual numbers of serious adverse events following receipt of a newly licensed vaccine. In 2005, the FDA responsibly issued a public advisory to physicians and parents after five (5) cases of Guillain Barre Syndrome (GBS) following receipt of Menactra by 17 and 18 year old girls occurred. A rough comparison of Gardasil and Menactra adverse event reports to VAERS through November 30, 2008 reveals that:
- Compared to Menactra, receipt of Gardasil is associated with at least twice as many Emergency Room visit reports; 4 times more Death reports; 5 times more “Did Not Recover” reports; and 7 times more “Disabled” reports.
- Compared to Menactra, receipt of Gardasil is associated with all of the reports of Blood Clots. All 23 reports of Blood Clots following Gardasil occurred when Gardasil was given alone without any other vaccines.
- Compared to Menactra, receipt of Gardasil is associated with at least 4 times as many Cardiac Arrest reports. All 9 reports of Cardiac Arrest following Gardasil occurred when Gardasil was given alone without any other vaccines.
- Compared to Menactra, receipt of Gardasil is associated with at least 6 times as many Fainting reports and at least 3 times as many Syncope reports.
- Compared to Menactra, receipt of Gardasil is associated with at least 4 times as many Lupus reports. 27 reports of Lupus following Gardasil occurred when Gardasil was given alone
- Compared to Menactra, receipt of Gardasil is associated with at least 15 times as many Stroke reports. 16 reports of Stroke following Gardasil occurred when Gardasil was given alone.
- Compared to Menactra, receipt of Gardasil is associated with at least 3 times as many Syncope reports.
- Compared to Menactra, receipt of Gardasil is associated with at least 33 times as many Thrombosis reports. 34 reports of Thrombosis following Gardasil occurred when Gardasil was given alone.
- Compared to Menactra, receipt of Gardasil is associated with at least 5 times as many Vasculitis reports. 11 reports of Vasculitis following Gardasil occurred when Gardasiil was given alone.
- Compared to Menactra, receipt of Gardasil is associated with at least 30 times as many Rechallenge reports, which involve a worsening of symptoms experienced after previous receipt of Gardasil.
Conclusion:
In 2007, the National Vaccine Information Center issued three analyses of VAERS reports following receipt of Gardasil. In these reports, we warned that Gardasil appeared to be associated with an unusually high number of reports of atypical collapse (fainting and syncope) and other symptoms of brain and immune system dysfunction.
A rough analysis of adverse events reported to VAERS following receipt of Gardasil and/or Menactra vaccines through November 30, 2008 indicate that Gardasil is involved in a much higher number of serious adverse health events than Menactra.
Although Gardasil is given in a three- shot series and only one dose of Menactra is given, Menactra is given to both boys and girls while Gardasil is given only to girls. It is unusual for there to be such a big discrepancy between two vaccines used in similar populations involving serious and relatively rare life threatening adverse events and autoimmune disorders such as death, blood clots, cardiac arrest, lupus, thrombosis, stroke, and vasculitis.
Fainting, which has been attributed by doctors and health officials as “fear” of needles in teenage girls is reported six times as often (and Syncope is reported three times as often) after receipt of Gardasil than Menactra even though Menactra is also given to girls in the same age group.
In pre-licensure clinical trials, Gardasil was only tested in fewer than 1200 girls 16 years and younger. Through November 30, 2008, in girls 16 or younger, there were reports of 9 deaths; 3 blood clots; 4 cardiac arrests; 9 cases of lupus; 6 strokes; and 2 cases of vasculitis developing after receipt of Gardasil.
This VAERS analysis gives compelling evidence for the need for action to be taken by the FDA, CDC and Congress:
- the FDA should further investigate reports of serious health problems and deaths following Gardasil vaccination; review the accuracy of information about adverse events contained in product manufacturer inserts; and inform physicians and parents about all serious health problems that have been reported to VAERS after Gardasil vaccination;
- the CDC should re-investigate VAERS reports of serious health problems and deaths after Gardasil vaccination; consider the need to withdraw the recommendation that all girls between the ages of 9 and 26 should receive Gardasil vaccine; and issue a warning that, when a serious adverse event occurs after Gardasil vaccination, no further Gardasil shots should be given;
- physicians in the fields of pediatrics and obstetrics/gynecology should fully inform patients and parents about all reported Gardasil adverse events and refrain from re-vaccinating those who experience serious health problems following Gardasil vaccination;
- Merck and the NIH should separately conduct studies into the biological mechanisms for Gardasil vaccine injury and death and define them for physicians and the public so: (a) biological high risk factors can be identified to facilitate informed medical decisionmaking ; (b) pathological profiles can be developed to confirm Gardasil- induced brain and immune system dysfunction and death; (c) healing therapies to moderate Gardasil- induced brain and immune system dysfunction can be developed; and (d) Merck can improve the safety of Gardasil;
- Congress should investigate the fast- tracking of Gardasil vaccine without adequate long- term safety studies in American pre-adolescent and teenage girls between ages 9 and16 and the safety and effectiveness of Gardasil vaccine in all age groups.
Vaccines which are licensed and recommended by the government for universal use by children and young adults, should adhere to the highest standards with regard to proof of safety and efficacy. In October 2008, the government issued a report maintaining that receipt of Gardasil is not associated with more serious health problems, hospitalizations, injuries and deaths among young girls and women than are experienced by those young girls and women who do not receive Gardasil. This analysis comparing adverse events reports to VAERS following receipt of Gardasil and Menactra appears to contradict that assertion.
Immediate action should be taken now by federal health agencies to protect recipients of Gardasil from injury and death.
Human Papillomavirus (HPV) & HPV vaccine quick facts
Quick Facts
HPV

- Human papillomavirus (HPV) is the most commonly sexually transmitted infection in the U.S. and there are more than 200 known HPV types, the majority of which are not harmful. About 75 percent of HPVs have been associated with non-cancerous warts (papillomas) on the hands, chest, arms and feet, such as low-risk HPV types 6 and 11;
- About 40 HPV types in the surfaces of the cervix, vagina, vulva, anus, penis, mouth and throat, and include the most common high-risk cancer-causing HPV types 16 and 18. High-risk HPV types are associated with development of cancer of the cervix and five other genital and oral cancers affecting women and men;
- Most sexually active women and men become infected with HPV and over 90 percent clear the infection within two years. Antibodies from prior infections help prevent future re-infection by the same HPV type. Chronic infections are rare and occur mostly in women. Chronically infected women who don’t get pre-cancerous cervical lesions promptly identified and treated can develop cervical cancer and die. High risk factors for developing HPV-related cancers include: smoking, multiple sexual partners, long-term oral contraceptive use, multiple births, weakened immune system, co-infection with chlamydia or HIV, poor nutrition, heavy drinking and smoking, and chronic inflammation;
- After Pap smear tests that screen for cancerous conditions became a routine part of gynecological health care for American women in the 1960’s, U.S. cervical cancer cases dropped by 75 percent. Women are recommended to get regular Pap tests throughout their life whether or not they get HPV vaccinations;
- According to the CDC, approximately 47,199 HPV-related cancers occur each year in the U.S. which represents less than three percent of all cancers diagnosed in the U.S. and one one hundredth of a percent (.0001) of the U.S. population. In 2023, the American Cancer Society estimates that 13,960 cases and 4,310 deaths will occur in the U.S.
HPV Vaccine
- Gardasil 9 by Merck was licensed in 2014 to prevent cervical, vulvar and anal cancers caused by high risk HPV types 16, 18, 31, 33, 45, 52, and 58; genital warts caused by low risk HPV types 6 and 11; and precancerous lesions caused by all of these HPV types. The vaccine is approved for use by females and males ages 9 to 45 years. The CDC recommends two doses of Gardasil 9, the only Food and Drug Administration (FDA) approved HPV vaccine currently available in the US, at age 11-12. Three doses of the vaccine are recommended if the vaccine is initially administered at age 15 through 26 years, and for those with immunocompromising conditions. Adults 26 through 45 years may choose to receive HPV vaccine, however, public health officials have acknowledged that the benefit to vaccination in this population is minimal.
- To initially evaluate safety, rather than uniformly comparing Gardasil to an inert saline placebo, the vaccine was compared to its bioactive aluminum adjuvant component. Later, the majority of Gardasil 9 clinical trial results were bootstrapped by comparing the new Gardasil 9 vaccine to the old Gardasil vaccine.
- Licensed in 2006 for 11-12 year old girls and young women, thousands of adverse reaction reports related to Gardasil were filed for: sudden collapse with unconsciousness within 24 hours, seizures, muscle pain and weakness, disabling fatigue, Guillain Barre Syndrome (GBS), facial paralysis brain inflammation, rheumatoid arthritis, lupus, blood clots, premature ovarian failure, optic neuritis, multiple sclerosis, strokes, heart and other serious health problems, including death were reported. Similar reports have been filed for the Gardasil 9 vaccine licensed in 2014 for use by females and males ages 9 to 45 years, even though the recommended number of doses was reduced from three to two.
- Using the MedAlerts search engine, as of June 30, 2023, the federal Vaccine Adverse Events Reporting System (VAERS) contains more than 74,229 reports of HPV vaccine reactions, hospitalizations, injuries and deaths and includes 628 related deaths, 7,484 hospitalizations, and 3,578 disabling conditions. Over 55 percent of the reported serious adverse events occurred in children and teens under 17 years of age. As of August 1, 2023, 822 claims were filed with the federal Vaccine Injury Compensation Program (VICP) for injuries and deaths following HPV vaccination, which included 22 deaths and 800 serious injuries. Of that number 169 claimants have received compensation.
Food & Drug Administration (FDA)
- Human Papillomavirus (HPV) Vaccine Product Inserts & Licensing Information
Centers for Disease Control (CDC)
- CDC on HPV
- CDC on HPV Vaccines & HPV Vaccine Safety
- CDC HPV Vaccine Information Statement (VIS) Gardasil 9
- CDC HPV Fact Sheet
Vaccine Reaction Symptoms & Ingredients
Our Ask 8, If You Vaccinate webpage contains vaccine reaction symptoms and more.
Search for Vaccine Reactions
NVIC hosts MedAlerts, a powerful VAERS database search engine. MedAlerts captures symptoms, reactions, vaccines, dates, geographic locations and more.
Reporting a Vaccine Reaction
Since 1982, the NVIC has operated a Vaccine Reaction Registry, which has served as a watchdog on VAERS. Reporting vaccine reactions to VAERS is required by law. If your doctor will not report a suspected vaccine reaction, you have the right to report it yourself.
IMPORTANT NOTE: NVIC encourages you to become fully informed about HPV and the HPV vaccine by reading all sections in the Table of Contents , which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
Additional bibliography of references
Select Broadcast Media Reports on Gardasil Vaccine
- Aug. 19, 2009. “Gardasil Researcher Speaks Out,” a report by Sharyl Attkisson, CBS-TV.
- Nov. 11, 2008. “One Mother’s Story & Bigger Questions About the U.S. Vaccine Safety System,” an investigate report by Jane Miller, WBAL-TV 11 (Baltimore).
Selected Print Media Reports
- The Pharmaceutical Journal Trials of HPV vaccine may have overestimated its efficacy, study finds Royal Pharmaceutical Society Feb. 4, 2020
- Cluskey P. Sharp drop in number of Dutch teenage girls getting HPV vaccination. The Irish Times. Jun. 27, 2018.
- Sagonowsky E. Merck CEO: Gardasil plus pipeline equals big things ahead for vaccines. FiercePharma. Jun. 12, 2018
- Llamas M. Florida Mom Says HPV Vaccine Ruined Daughter’s Life. Drugwatch. May 15, 2018.
- Pulla, P. Understanding the HPV vaccine’s risk. The Hindu. Feb. 5, 2018.
- Joelving F. What the Gardasil Testing May Have Missed. Slate. Dec. 17, 2017.
- Cullen P. Almost 650 girls needed medical intervention after HPV vaccine. The Irish Times. Sep. 12, 2017.
- Guevara C. Class Action Lawsuit Against HPV Vaccine Filed in Colombia. Medscape. Aug. 7, 2017.
- Aoki, M. Suit opens in Tokyo court over cervical cancer vaccine side effects. The Japan Times Feb. 13, 2017
- Chustecka Z. Class Action Lawsuit Against HPV Vaccine Filed in Japan. Medscape. Jul. 27, 2016.
- Chustecka Z. Complaint Filed Over EMA's Handling of HPV Vaccine Safety Issues. Medscape. Jul. 5, 2016
- Chustecka Z. Case Reports of 'Syndrome' Appearing After HPV Vaccination. Medscape. Sep. 18, 2015
- Gallagher P. Thousands Of Teenage Girls Report Feeling Seriously Ill After Routine School Cancer Vaccination. The Independent. May 31, 2015.
- Dennis B. Five pressing health priorities in 2014. Washington Post. Dec. 28, 2013.
- Sanofi sued in France over Gardasil vaccine. Reuters. Nov. 24, 2013.
- Baklinski T. Japan Withdraws Support of Controversial HPV Vaccine Over Safety Concerns. Lifesite News. Oct. 16, 2013.
- Mulcahy, N. Japan Withdraws HPV Vaccine Recommendation for Girls. Medscape. Jun. 25, 2013.
- Castillo M. Side effect fears stop parents from getting HPV vaccine for daughters. CBS News. Mar 18, 2013.
- Gonthier V. Family Sues After Teen Dies Following HPV Vaccination. Toronto Sun. Jan. 31, 2012.
- Green G. Gardasil: Krystal’s Story. Women Hurt by Medicine. May 14, 2010.
- Scoop Independent News (NZ). Vaccine Roll-Out Is Wrong Without Honest Advice. Feb. 28, 2009.
- Hart, B. “Gardasil Vaccine for boys?” Seattle Post Intelligencer . Jan. 16, 2009.
- “FDA Again Says No to Expanded Gardasil Use”. Wall Street Journal. Jan. 9, 2009.
- Edelman S. Fed’s Warning Shot. New York Post. Jul. 6, 2008.
- “My Girl Died as ‘Guinea Pig’ for Gardasil” New York Post. Jul. 20, 2008.
- “Is HPV Vaccine To Blame for Teen’s Paralysis?” US News & World Report. Jul. 2, 2008.
- “Judicial Watch Uncovers New FDA Records Detailing Ten New Deaths & 140 “Serious” Adverse Events Related to Gardasil.” Judicial Watch. June 30, 2008.
- “Anti-Vaccine Activists vs. Gardasil”. Time Magazine. Jun. 19, 2008.
- UCSF Doctors Warn on Wide Use of Cervical Cancer Vaccine. San Francisco Chronicle. May 10, 2007.
- “Merck posts 12% profit rise, affirms 2007 forecast”. Market Watch. Apr. 19, 2007.
- “VA set to Mandate Gardasil”. Boston Globe. Mar. 3, 2007.
- Drugmaker Assists In Pushing for Mandate For HPV. Washington Post. Feb. 11, 2007. .
- Letter to the Editor from Barbara Loe Fisher, “Risk, Racism and the HPV Vaccine”. Washington Post. Feb. 1, 2007.
- “Force Is Not the Only Way to administer a Vaccine” by Courtland Milloy. Washington Post. Jan. 24, 2007.
- D.C. Bill Would Mandate Vaccine. Washington Post. Jan. 10, 2007.
- “A New Vaccine for Girls, But Should It Be Compulsory?” by Roni Rabin. New York Times. Jul. 18, 2006.
Selected Medical Literature Articles
- Shiller JT, Lowy DR. Papillomavirus-Like Particle Vaccines. J Natl Cancer Inst. Monogr 2000; 28: 50-54.
- Baylor NW, Egan W, Richman P. Aluminum salts in vaccines – US perspective. Vaccine May 31, 2002; 20Supple 3: 518-523.
- Burd EM. Human Papillomavirus and Cervical Cancer. Clin Microbiol Rev 2003; 16(1): 1-17.
- Haug CJ. Human Papillomavirus Vaccine: Reason for Caution. NEJM 2008; 359: 861-862.
- Slade BA, Leidel L, Vellozzi C, Woo EJ et al. Postlicensure Safety Surveillance for Quadrivalent Human Papillomavirus Recombinant Vaccine. JAMA 2009; 302(7): 750-757.
- Sutton I, Lahoria R et al. CNS Demyelination and Quadrivalent Vaccination. Mult Scler 2009; 15: 116-119.
- Rothman SM, Rothman DJ. Marketing HPV Vaccine: Implications for Adolescent Health and Medical Professionalism. JAMA 2009; 302(7): 781-786.
- Haug C. The Risks and Benefits of HPV Vaccination. JAMA 2009; 302(7): 795-796.
- Alvarex-Sonia MJ, Hernandez-Gonzalez A et al. Demyelinating Disease and Vaccination of the Human Papillomavirus. Rev Neurol 2011; 52(8): 472-476.
- Souayah N, Michas-Martin PA. Guillain Barre syndrome after Gardasil vaccination: data from Vaccine Adverse Events Reporting System 2006-2009. Vaccine 2011; 29(5) 886-889.
- Klein NP, Hansen J et al. Safety of Quadrivalent Human Papillomavirus Vaccine Administered Routinely to Females. JAMA Pediatrics 2012; 166(12): 1140-1148.
- Soldevilla HF, Brones SFR, Navarra SV. Systemic lupus erythematosus following HPV immunization or infection?. Lupus Feb. 2012; 21: 158-161.
- Van Bogaert LJ. Are the currently existing anti-human papillomavirus vaccines appropriate for the developing world? AMHSR 2013; 3(3): 306-312.
- Pons-Salort M, Tiebault ACM et al. HPV genotype replacement: too early to tell. The Lancet Infectious Diseases 2013; 13(12): 1012.
- Arnheim-Dahlstrom L, Pasternak B et al. Autoimmune, neurologic, and venous thromboembolic adverse events after immunization of adolescent girls with quadrivalent human papillomavirus vaccine in Denmark and Sweden: cohort study. BMJ 2013; 347
- Mariani L, Venuti A. HPV vaccine: an overview of immune response, clinical protection, and new approaches for the future. Journal of Translational Medicine 2010; 8:105.
- Colafranceso S Perricone C et al. Human Papilloma Virus Vaccine and Primary Ovarian Failure: Another Facet of Autoimmune/Inflammatory Syndrome Induced by Adjuvants. American Journal of Reproductive Immunology 2013.
- Binagwaho A, Wagner CM et al. Achieving a high coverage in Rwanda’s national human papillomavirus programme. Bulletin of the World Health Organization 2012; 90: 623-28.
- Batson A, Meheus F, Brooke S. Chapter 26: Innovative financing mechanisms to accelerate the introduction of HPV vaccines in developing countries. Vaccine Aug 31, 2006;24 Suppl 3:S3/219-25.
- Gupta S., Kerkar R A., et al. Is human papillomavirus vaccination likely to be a useful strategy in India? South Asian J Cancer. 2013 Oct-Dec; 2(4): 193–197.
- Tomljenovic, L., Colafrancesco, S., et al. Postural Orthostatic Tachycardia With Chronic Fatigue After HPV Vaccination as Part of the “Autoimmune/Auto-inflammatory Syndrome Induced by Adjuvants” Case Report and Literature Review. J Investig Med High Impact Case Rep. 2014 Jan-Mar; 2(1): 2324709614527812.
- Little, D.T., Ward, H.R., Adolescent Premature Ovarian Insufficiency Following Human Papillomavirus Vaccination: A Case Series Seen in General Practice. J Investig Med High Impact Case Rep. 2014 Oct 28;2(4):2324709614556129.
- Palmieri B, Piddighe D et al. Severe somatoform and dysautonomic syndromes after HPV vaccination: case series and review of literature. Immunol Res 2017; 65(1): 106-116.
- Chandler RE, Juhlin K et al. Current Safety Concerns with Human Papillomavirus Vaccine: A Cluster Analysis of Reports in VigiBase. Drug Safety 2017; 40_ 81-90.
- Chandler, R.E. Safety Concerns with HPV Vaccines Continue to Linger: Are Current Vaccine Pharmacovigilance Practices Sufficient? Drug Saf. 2017; 40(12): 1167–1170.
- Cervantes LJ, Doan AH. Discrepancies in the evaluation of the safety of the human papillomavirus vaccine. Mem Inst Oswaldo Cruz 2018; 113(8).
- Jørgensen L., Gøtzsche P.C., et al Index of the human papillomavirus (HPV) vaccine industry clinical study programmes and non-industry funded studies: a necessary basis to address reporting bias in a systematic review. Syst Rev. 2018; 7: 8.
- Rees CP, Brhlikova P, Pollock AM. Will HPV vaccination prevent cervical cancer? J R Soc Med. Jan 21, 2020:141076819899308.
National Institutes of Health (NIH)
- Biological Therapies for Cancer
- Cancer Stat Fact Sheets (National Cancer Institute)
American Cancer Society
- Can Cervical Cancer Be Prevented?
- Key Statistics for Cervical Cancer
- Key Statistics for Oral Cavity and Oropharyngeal Cancers
- Key Statistics for Vaginal Cancer
- Key Statistics for Penile Cancer
- Key Statistics for Anal Cancer
- Key Statistics for Vulvar Cancer
- HPV and Cancer - What is HPV?
IMPORTANT NOTE: NVIC encourages you to become fully informed about HPV and the HPV vaccine by reading all sections in the Table of Contents , which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
Gardasil Vaccine & the Damage Done
Ashley and her Mom, Lisa
 |
| Ashley |
 |
|
Ashley, hospitalized after Gardasil shots |
Report a Vaccine Reaction to NVIC
VIDEO of Ashley talking about her Gardasil reaction
Read about similar Gardasil reactions reported to the government
Newspaper story about Ashley’s Gardasil reaction
Ashley’s Gardasil Reaction Story
by her Mom, Lisa
December 2008
My daughter received two Gardasil shots, one in August and one in October 2007 along with the vaccine for meningitis.
Since then, my healthy teenage daughter became a chronically ill young woman. In the last year I have called 911 more times than I can count. She has been hospitalized three times within a year. We have seen many doctors and specialists.
Ashley experiences life threatening episodes of seizure like activity, difficulty breathing, major back spasms, paralysis, dehydration, memory loss and tremors. At times she becomes unresponsive. She has daily illnesses of nausea, exhaustion, vomiting, pain, and hair loss. I have provided CPR to her on a regular basis.
Ashley has missed a semester of school and now only attends 1-2 days a week. She has no energy to have a normal teen life and keep up with her friends. She can sleep up to 15 hours a day. After an episode she will sleep up to three days.
Before this, Ashley was a healthy girl, involved in extra curricular school activities and has always received top grades.
In December 2008 we accepted the fact that she has been having a vaccine reaction. For the last year I have been researching her symptoms and it was Ashley who asked me about the vaccines. I was shocked as I researched and read stories of other young women having the same experiences as Ashley.
Having a chronically ill child for over a year and not knowing why has been unreal. Now that I know why this is happening to her, feel even more perplexed. We have no answers or treatment.
Our primary care doctor, who has been very kind, has spent the time researching Ashley’s four inch high medical records, admits this is out of his area of expertise. He has no idea who to refer us to. We are treating her symptoms with pain killers and medication for nausea and vomiting.
It is unacceptable to have my 16 year old daughter on a strong addictive pain killing drug. However, it is all she has to help her cope with her severe symptoms.
I have learned some parents are seeking treatment for their daughters from doctors who practice alternative health care. I have made multiple appointments with different alternative medical doctors with the hope that we find one who will take the time to educate us and provide treatment that is safe and works.
My first question I have will be, “How can we detox the body of a vaccine that was made to protect her for life?” Regular or primary doctors have warned me to be careful with alternative medicine due to that could cause more damage to her body. At the same time her regular doctors do not know what to do for her either.
Ashley came into my life when I was 20. We grew up along side each other. I am a single mother and have dedicated my life to provide a safe, fun, loving environment for my daughter. She has been a gift in my life. I describe her as having an old soul. People of all ages, race, disabled, even animals connect with her. She has always attracted attention from everyone even complete strangers. Ashley will immediately help anyone in need - from holding open a door to standing up for a child being picked on.
People in our community always tell me what a great job I have done raising her. The truth is, she was born with this kindness. And because of who Ashley is, the people, community, schools, and family have always been very supportive. I have never felt I raised my child alone. I am so proud of her.
Now Ashley is sick. Her life is dealing with daily symptoms and hoping for a day without a life threatening episode. She is trying to go to school and stay caught up with her school work. Her teachers, counselors, school nurse and administrators are supporting her and becoming personally involved with her education. They have gone above and beyond what I can ask for. Even taking the responsibility to provide the skills necessary to keep her stable when she has an episode at school before the ambulance arrives.
Ashley and I have had conversations in the event if she may die. I can only promise her that I will do everything in my power for this not to happen. And we need to focus on healing. I never thought in a million years a vaccine would do this much damage to my beautiful girl.
We are struggling financially now because of the medical bills. I have always provided the basic needs and stayed off government help. Ashley deserves to have the best medical treatment she can get so she can heal. I don’t know what is going to happen to her, to us.
I stay strong for my daughter. I also cry with her too. I keep faith in knowing God doesn’t put more on our shoulders than we can handle. As a mother, I also feel the responsibility to get Ashley’s story out there so we can help other families.
Gardasil Vaccine & the Damage Done
Cassie, 15
 |
| Cassie |
Report a Vaccine Reaction to NVIC
Read about similar Gardasil reactions reported to the government
"Our Gardasil Nightmare"
by Cassie's Mom, Kim
Our nightmare started in December 2007 without us even knowing it when my fun loving, active daughter willingly pulled up her sleeve and received the first of three Gardasil shots. Cassie, 14 at the time, is known for her genuine love of life and her heart-warming smile. She was very active in her church and youth group and was an athlete, playing volleyball and running track. That was until Gardasil vaccine stole her life away from her.
After receiving the first Gardasil shot, she started experiencing severe headaches and nosebleeds, which continued after the second shot and increased in severity and added to her extreme fatigue. After seeing our family physician twice and still not connecting her health problems to Gardasil, Cassie received the third Gardasil shot as well as other vaccines.
That's when everything fell apart. The nose bleeds were severe and the headaches at times were so bad she would hold her head and cry. The fatigue left her unable to move and, then, the flu-like symptoms and severe abdominable pain hit her.
Since receiving her third Gardasil shot, Cassie has been hospitalized four times. She has been diagnosed with pancreatitis, gastrointestinal disorders, and has had pneumonia three times. She is also suffering from various other symptoms including extreme nausea and vomiting after having a central line put in and being on TPN and Lipids (IV nutrition). She has fatigue, dizziness, headaches, hypersensitivity to light and sound, blurred vision, lactation, seizure-like activity, tingling and numbness in legs, and severe pain in her abdomen.
My once fun-loving, active daughter is now debilitated with illness and pain. However, she refuses to allow Gardasil to take away her loving spirit and warm smile. Through all of this she has taught me so much about never giving up and never losing faith in God. Even at times when I was so afraid to take my eyes off her in fear she would not take another breath, she would say "I know God has a plan I know there is a reason for my sickness."
We could all learn a lot from these girls. I know I have. I am so proud of Cassie.
Gardasil Vaccine & the Damage Done
Christina Tarsell and Emily, her mother
 |
| Emily Tarsell in front of a photo of Chris with her Dad |
 |
| Christina Tarsell, 21 years old |
 |
| Painting by Chris 2007 |
Report a Vaccine Reaction to NVIC
Watch a VIDEO with Emily talking about Christina’s Gardasil reaction (8 minutes)
Read about similar Gardasil reactions reported to the government
Christina Tarsell was a 21-year-old college student majoring in studio arts at Bard College when she received a series of three Gardasil shots. A talented athlete, artist and honor roll student, she died suddenly and without explanation shortly after the third shot in June 2008. Her mother Emily Tarsell, who is gathering information on Gardasil vaccine reactions as NVIC’s Director of Gardasil Network Development, writes on the website she has created for Christina (www.Gardasilandunexplaineddeaths.com):
“When Chris wasn't in the studio painting, constructing or writing, or at the gallery working, Chris liked to hang out with her friends. Chris would have been a senior in studio arts at Bard College, a community she loved. She liked exchanging ideas, observing nature, listening to music, debating, dancing, cooking, and laughing with her companions.
Frequently, she and friends would take excursions to New York City where she did an internship. They explored museums, galleries, and neighborhoods like Chinatown, Brooklyn and Williamsburg. It was all so exciting and the future looked rich with possibilities. But it all came to an abrupt end when Chris was found dead on June 23 at home in Tivoli, NY in a house she shared with several other college students. Chris was 21.
Born and reared in Sparks, Maryland, Chris was an honor student, a member of NHS, Amnesty International, The Towson Unitarian Universalist Church, and Girl Scout Troop 589 who presented her with a Gold Award. An avid and accomplished athlete, Chris played baseball on the boy’s team in middle school, varsity softball in high school and tennis in college. She was art editor of two literary magazines, Brillig and Verse Noire, in high school and college, respectively.
Her passion was art and she won several juried art honors. She loved texture, light and color and was just embarking on a serious and challenging senior project integrating sculpture and painting when her life tragically ended. Sweet, spirited, questioning, generous and caring, she is deeply missed. We love you Chris.”
Gardasil Vaccine & the Damage Done
Gabrielle and her mother, Shannon
 |
| Gabrielle with her sister |
 |
| Gabi in the Emergency Room after a collapse, trying to keeping her spirits up |
Watch a VIDEO “Gardasil: The Damage Done from a Best Friend’s View”
Watch a VIDEO with Shannon & Gabi talking about her Gardasil reaction (6 minutes)
Report a Vaccine Reaction to NVIC
Read about similar Gardasil reactions reported to the government
Gabrielle’s Gardasil Vaccine Reaction
by Shannon, her Mom
December 2008
Gabi received three Gardasil shots in November 2007 and February and June 2008.
Gabi was a healthy, strong teenage girl prior to her first shot of Gardasil. She was the epitome of the All-American girl…beautiful, smart, and caring with a heart of gold. She was a varsity and competitive All-Star cheerleader, gymnast, Honor Roll student involved in leadership and sang in a Bel Canto Choir at her high school.
Gabi had taken a vow to wait for the man she would spend the rest of her life with and she had many dreams she was going to fulfill before she got married. There is cervical cancer in our family and we both wanted to do everything possible to protect her from getting cervical cancer someday. That is why we decided to have her get the series of Gardasil shots.
Immediately following her first shot of Gardasil, her arm went numb and remained that way for several minutes. Within a week of receiving her first shot, Gabi’s life began to change.
On December 4, 2007, she experienced the first of many symptoms involving severe muscle fatigue. Gabi began developing conditions that were unexplainable: excessive fatigue; headaches; muscle weakness and pain; joint weakness and pain; a rash that resembled a sunburn; loss of concentration; and numbness and tingling in her hands and feet. During this time we were completely unaware that these things were side-effects of her recent Gardasil shot.
Still not knowing what was going on with Gabi’s body, she received her 2nd vaccine in February 2008. Immediately following this shot, her symptoms got worse. Her grades started dropping. She became more irritable. Her condition deteriorated. She began to “shiver” for no apparent reason.
The doctors started looking for answers and giving us conflicting advice like she needed to condition her body more, she needed to slow down or she was a normal teenager. We were at a loss. She had been a gymnast since she was four years old and had been in perfect health and shape.
In June 2008 Gabi reminded me that she needed to get the 3rd shot in the Gardasil series. I scheduled her appointment and took her in for that last shot, both of us convinced we were doing something that was going to save her from getting a horrible disease someday.
Again, immediately following the shot her arm went numb. This time, she complained that she was dizzy.
In the weeks following, Gabi’s condition progressively got worse. She spent more time feeling sick and without feeling in her hands and feet. She was swelling in her face, hands and feet when she woke up in the morning and would stay that way for several hours. Her menstrual cycles had become extremely heavy and irregular. Gabi began losing hair by the handfuls. Her body ached the majority of the time. The doctors could give us no explanation except that she was a typical teenage girl and probably needed to cut back on her activities.
In September 2008, Gabi collapsed from an episode of severe chest pains and shortness of breath. She insisted that it was because she had overworked herself at the gym. Two days later she collapsed with the same symptoms: severe chest pains and shortness of breath. She once again made light of it, thinking it was nothing more than dehydration from an intense workout at the gym.
Two days later we were driving her to a football game where she was supposed to cheerlead when she began complaining the she couldn’t breathe and was in severe pain in her chest. We rushed her to the emergency room, for the first on many trips to the hospital. They examined her thoroughly and, despite the rapid, irregular heart beat and low oxygen levels, they sent her home saying that they had no explanation as to why my daughter was exhibiting heart attack like symptoms.
Gabi was seen a total of four more times at the hospital the same weekend, with the same result. This was just the beginning of a horrific battle to try and get answers as to what was happening to her. It was a painful time of finding someone who was willing to help her and not tell her and me that she was crazy.
Gabrielle’s condition is progressively getting worse. The symptoms she is currently dealing with on a daily basis are as follows:
Excessive fatigue
Loss of concentration
Difficulty with speaking
Memory loss
Muscle weakness and pain
Joint weakness and pain
Dizziness
Loss of appetite
Weight loss
Severe headaches
Severe chest pain
Shortness of breath
Difficulty breathing
Nausea and vomiting
Recurring rash
Tingling and burning in hands and feet
Severe sensation of burning throughout the body
Tremors
Hair loss
Abdominal pain
Partial paralysis
Partial loss of vision
Seizures daily, non-responsive to medication
Transient Ischemic Attacks, or “mini-strokes”
Gabrielle is taking numerous medications several times a day to try and control what is happening to her body. At 15 years old, she is on a beta-blocker for heart problems; aspirin to try and prevent another stroke; medication to help with the severe headaches; and several medications to try and control the seizures. Gabi will tell you that she is a 15 year old girl trapped in the body of an 80 year old woman.
After unsuccessfully attempting to go back to school in wheelchair, Gabi has been out of school since October 2008 with the exception of a few hours here and there trying to go to school. She has been placed on a home-based program. The administration, staff and teachers of her high school have been absolute angels, providing support and help not only academically but personally as well. They have gone above and beyond what we could have ever dreamed of or expected.
Her struggle to maintain passing grades increases daily and she fears that her chance of a college education is slipping away. She has lost almost all social interaction with her friends and has learned a hard lesson about friendship. She knows she has changed and is different but she continues to smile and treat her friends the same even if some of them don’t treat her the same.
Gabrielle is currently under the care of pediatric neurologists. She has been diagnosed with Inflammation of the Central Nervous System as a result of a Gardasil vaccine reaction. Her prognosis is not good. Without a medication or something that can be done to stop or modify what this vaccine is doing to her body, we have been told she will die. The progressive deterioration has been rapid.
We are pleading for help and answers. We pray for a miracle.
Gabrielle has come to terms with the fact that she may not make it. Her hope and prayer is that she will make a difference in the lives of others and keep this from happening to another girl. If you were to ask her if she had the opportunity to ask just one question, what would it be? Her reply would be “WHY?”
Gabi has been robbed of a normal life. She may never have the opportunity to achieve her dreams. I may never get the opportunity to watch her be all she can be as a young woman, a college student, a wife and a Mom. All of this because of a vaccine that was intended to save lives of millions, a vaccine that was “fast-tracked” and lacked long term safety studies.
Thousands of girls are reporting they are suffering side-effects from the Gardasil vaccine. Ask any one of those parents with a daughter suffering if this is what they wanted for their daughter. Ask any one of those parents if they were warned this could happen. Ask any of those parents if they knew they were risking their daughter’s life to possibly avoid getting a cancer that is almost 100 percent preventable with routine pap exams and is 100 percent curable if identified and caught early.
Gabi’s life has been forever changed. Neither of us had the information we should have had before she got vaccinated. Without help Gabi will never have a chance to live the life she so much deserves.
Gardasil Vaccine & the Damage Done
Megan and her mother, Karen
 |
| Megan with her nephew at football practice |
 |
| Megan |
Report a Vaccine Reaction to NVIC
Read about similar Gardasil reactions reported to the government
Megan was a healthy, bright 20 year old college student who did not smoke or drink and got regular medical check-ups. She wanted to work in the health care field and was engaged to be married after she graduated from college.
Megan got a third Gardasil shot in September 2008. In the days and weeks following the shot, she said she felt very tired and was having a lot of “dizzy spells.” On November 15, 2008, she died suddenly without explanation. No cause of death was found.
Megan loved to look at the stars at night. Just before Christmas, her family spread her ashes on Coal Bank Pass in Colorado at 11,000 feet so she could be close to the stars she loved.
Letter to Megan from Karen
January 2009
Dear Megan,
I am so sorry that I can't talk with you anymore. I miss you so much. I had spoken with you only hours before that day in November and I can’t believe you are gone.I can't help but sit here and think about you and what you would be doing if you were still here.
You were only twenty years old, would have been twenty one on Thanksgiving Day. You had so many dreams. You were studying Radiology in college. Watching babies in the womb was something you had really wanted to do. We will never know what you would have done with your life, how far you would have gone.
We won't have the chance to see you graduate or be a bride. I know how much your sisters were looking forward to making you the most beautiful bride ever. The day won’t come when I will watch you hold your own child in your arms. I remember watching you from a distance with your nieces and nephew and thinking of the Mom that you would be. Now I will never know.
Your 16 year old brother cries for you and I don't know what to do but to hold him and cry with him. How can we tell him that this vaccine that is advertised on T.V. and is so highly recommended in the doctors offices as something that is going to protect our children is actually taking lives and making others so ill. How can we ask him to trust what doctors say?
We thought that getting the Gardasil vaccine was the right thing to do. We thought it would protect you, so that you would be here to live all those dreams.
It was only weeks after you had the third shot that you died suddenly, without warning and with no answers. Now you are gone and so are all the dreams.
We are going to miss you terribly. My heart is breaking writing you this letter.
With all my love,
Mom
Gardasil Ruined my Daughter's Life
Victoria, 17
 |
| Victoria |
Report a Vaccine Reaction to NVIC
Read about similar Gardasil reactions reported to the government
Gardasil Ruined my Daughter's Life
by Victoria's Mom, Jodi
My daughter, Victoria, has been ill since February 2008. She had her first Gardasil vaccination in November 2007. Her second vaccination was in the beginning of February 2008. Immediately after her second vaccination, Victoria experienced severe diarrhea and was nauseous for about eight weeks. She had blood work done many times by doctors looking for a virus. On March 31, 2008, Victoria had her first seizure and was seen by neurologists, none of whom related her seizures to Gardasil. Meanwhile, I started to do research on the internet and found hundreds of families with daughters who have experienced the exact same symptoms my daughter experienced after Gardasil vaccination and are sharing stories, symptoms and information.
Victoria currently experiences the following symptoms: non-epileptic seizures, migraines, fainting, tremors, twitches, numbness, intermittent leg paralysis and facial paralysis, tingling, staring or blank episodes, eye pain, joint pain, neck pain, back pain, memory loss, confusion, brain fog, regression, mood swings and chronic fatigue. She continues to have bouts of nausea and diarrhea. She has not been in school since April 2008. She can never be left home alone. She can't go to school, go out with her friends or work or has little "normalcy" in her life. She has very few good days and always says she doesn't feel good.
Victoria has had CT scans, MRI's, MRA's, EEG's, blood work and was hospitalized at an epilepsy center in the video EEG monitoring unit for two separate weeks in May 2008 and September 2008. She was put on many different seizure medications. After the normal EEG results, she was taken off all medications. Her SED rate has always been high and she does have protein in her urine, but doctors do not seem concerned. She has been seen by neurologists, a psychiatrist, psychologist, several neuropsychologists, an immunologist, several infectious disease doctors. She was treated at a Wellness Center for a period of time. Wellness Center physicians believe that my daughter may have had Lyme disease that was dormant until the Gardasil vaccine. Infectious disease doctors differ. Which doctors are correct? I have no idea.
I do not know which way to turn for help. We have seen so many doctors and I can't seem to find anyone willing to help my daughter. There are so many other young girls who have the same exact symptoms as my daughter . The one thing that all of the girls have in common is that they got one or more Gardasil shots and their health suddenly deteriorated and they were never the same again.
We are on a fixed income like most people. We have expended many thousands of dollars in an effort to seek medical opinions and assistance for our daughter. Although we do have medical insurance, it is very difficult to find doctors willing to treat my daughter who will accept our HMO.
Gardasil vaccine has been on the market for less than three years. There are few "traditional" medical doctors, who will relate my daughter's symptoms to Gardasil. I am told "there is not enough information available" about the vaccine and doctors believe it to be "safe." I have heard that there are a few doctors making the correlation between Gardasil and the symptoms my daughter and many others are experiencing. However, the only doctors I know of right now are in California and Kansas. Other doctors who take an holistic approach are willing to try alternative treatments, such as homeopathy, but alternative health therapies are often not covered by health insurance so it becomes very costly.
Each and every night, I check on my daughter many times in the middle of the night to make sure she is still breathing (like we ALL did when they were babies). I have a chime on her bedroom door so that every time she opens it, I know she has walked out of her room. I had a deadbolt put on the front door of our home with a key that can be removed from the inside. I never leave the key in the door for fear that Victoria will be confused after a seizure or when she has memory loss, and leave our home. (This has happened many times and she has been missing). When she is in the shower, I have to either stand outside the door and/or keep asking her "are you okay?"
We are in desperate need of medical treatment for my daughter. I have run out of ideas, doctors to treat her and our money is running out. I do not know which direction to turn. Why aren't more doctors speaking out and helping our daughters?
Each and every day, I cry and wonder if Victoria will be the next one to die from the side effects of Gardasil vaccine. How many more girls like my daughter will have their lives ruined by this vaccine?



